Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: A dose escalating phase I/II study
- PMID: 17060934
- PMCID: PMC2360729
- DOI: 10.1038/sj.bjc.6603437
Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: A dose escalating phase I/II study
Abstract
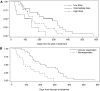
Patients with inoperable pancreatic cancer have a dismal prognosis with a mean life expectancy of 3-6 months. New treatment modalities are thus urgently needed. Telomerase is expressed in 85-90% of pancreas cancer, and immunogenic telomerase peptides have been characterised. A phase I/II study was conducted to investigate the safety, tolerability, and immunogenecity of telomerase peptide vaccination. Survival of the patients was also recorded. Forty-eight patients with non-resectable pancreatic cancer received intradermal injections of the telomerase peptide GV1001 at three dose levels, in combination with granulocyte-macrophage colony-stimulating factor. The treatment period was 10 weeks. Monthly booster vaccinations were offered as follow-up treatment. Immune responses were measured as delayed-type hypersensitivity skin reaction and in vitro T-cell proliferation. GV1001 was well tolerated. Immune responses were observed in 24 of 38 evaluable patients, with the highest ratio (75%) in the intermediate dose group. Twenty-seven evaluable patients completed the study. Median survival for the intermediate dose-group was 8.6 months, significantly longer for the low- (P = 0.006) and high-dose groups (P = 0.05). One-year survival for the evaluable patients in the intermediate dose group was 25%. The results demonstrate that GV1001 is immunogenic and safe to use. The survival data indicate that induction of an immune response is correlated with prolonged survival, and the vaccine may offer a new treatment option for pancreatic cancer patients, encouraging further clinical studies.
Figures





References
-
- Arai J, Yasukawa M, Ohminami H, Kakimoto M, Hasegawa A, Fujita S (2001) Identification of human telomerase reverse transcriptase-derived peptides that induce HLA-A24-restricted antileukemia cytotoxic T lymphocytes. Blood 97: 2903–2907 - PubMed
-
- Autexier C (1999) Telomerase as a possible target for anticancer therapy. Chem Biol 6: R299–R303 - PubMed
-
- Boyton RJ, Altmann DM (2002) Is selection for TCR affinity a factor in cytokine polarization. Trends Immunol 23: 526–529 - PubMed
-
- Burris III HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15: 2403–2413 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

