Differential effects of cigarette smoke on oxidative stress and proinflammatory cytokine release in primary human airway epithelial cells and in a variety of transformed alveolar epithelial cells
- PMID: 17062156
- PMCID: PMC1634855
- DOI: 10.1186/1465-9921-7-132
Differential effects of cigarette smoke on oxidative stress and proinflammatory cytokine release in primary human airway epithelial cells and in a variety of transformed alveolar epithelial cells
Erratum in
- Respir Res. 2008;9:6.
Abstract
Background: Cigarette smoke mediated oxidative stress and inflammatory events in the airway and alveolar epithelium are important processes in the pathogenesis of smoking related pulmonary diseases. Previously, individual cell lines were used to assess the oxidative and proinflammatory effects of cigarette smoke with confounding results. In this study, a panel of human and rodent transformed epithelial cell lines were used to determine the effects of cigarette smoke extract (CSE) on oxidative stress markers, cell toxicity and proinflammatory cytokine release and compared the effects with that of primary human small airway epithelial cells (SAEC).
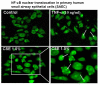
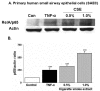
Methods: Primary human SAEC, transformed human (A549, H1299, H441), and rodent (murine MLE-15, rat L2) alveolar epithelial cells were treated with different concentrations of CSE (0.2-10%) ranging from 20 min to 24 hr. Cytotoxicity was assessed by lactate dehydrogenase release assay, trypan blue exclusion method and double staining with acridine orange and ethidium bromide. Glutathione concentration was measured by enzymatic recycling assay and 4-hydroxy-2-nonenal levels by using lipid peroxidation assay kit. The levels of proinflammatory cytokines (e.g. IL-8 and IL-6) were measured by ELISA. Nuclear translocation of the transcription factor, NF-kappaB was assessed by immunocytochemistry and immunoblotting.
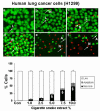
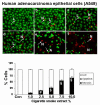
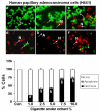
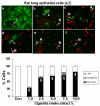
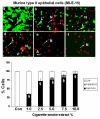
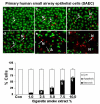
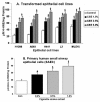
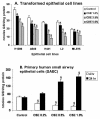
Results: Cigarette smoke extract dose-dependently depleted glutathione concentration, increased 4-hydroxy-2-nonenal (4-HNE) levels, and caused necrosis in the transformed cell lines as well as in SAEC. None of the transformed cell lines showed any significant release of cytokines in response to CSE. CSE, however, induced IL-8 and IL-6 release in primary cell lines in a dose-dependent manner, which was associated with the nuclear translocation of NF-kappaB in SAEC.
Conclusion: This study suggests that primary, but not transformed, lung epithelial cells are an appropriate model to study the inflammatory mechanisms in response to cigarette smoke.
Figures

References
-
- Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann NY Acad Sci. 1993;686:12–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

