A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer
- PMID: 17062687
- PMCID: PMC2154351
- DOI: 10.1158/1078-0432.CCR-06-1183
A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer
Abstract
Purpose: A phase I study was conducted to assess the safety of adoptive immunotherapy using gene-modified autologous T cells for the treatment of metastatic ovarian cancer.
Experimental design: T cells with reactivity against the ovarian cancer-associated antigen alpha-folate receptor (FR) were generated by genetic modification of autologous T cells with a chimeric gene incorporating an anti-FR single-chain antibody linked to the signaling domain of the Fc receptor gamma chain. Patients were assigned to one of two cohorts in the study. Eight patients in cohort 1 received a dose escalation of T cells in combination with high-dose interleukin-2, and six patients in cohort 2 received dual-specific T cells (reactive with both FR and allogeneic cells) followed by immunization with allogeneic peripheral blood mononuclear cells.
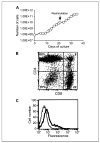
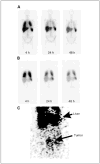
Results: Five patients in cohort 1 experienced some grade 3 to 4 treatment-related toxicity that was probably due to interleukin-2 administration, which could be managed using standard measures. Patients in cohort 2 experienced relatively mild side effects with grade 1 to 2 symptoms. No reduction in tumor burden was seen in any patient. Tracking 111In-labeled adoptively transferred T cells in cohort 1 revealed a lack of specific localization of T cells to tumor except in one patient where some signal was detected in a peritoneal deposit. PCR analysis showed that gene-modified T cells were present in the circulation in large numbers for the first 2 days after transfer, but these quickly declined to be barely detectable 1 month later in most patients. An inhibitory factor developed in the serum of three of six patients tested over the period of treatment, which significantly reduced the ability of gene-modified T cells to respond against FR+ tumor cells.
Conclusions: Large numbers of gene-modified tumor-reactive T cells can be safely given to patients, but these cells do not persist in large numbers long term. Future studies need to employ strategies to extend T cell persistence. This report is the first to document the use of genetically redirected T cells for the treatment of ovarian cancer.
Figures

References
-
- Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–21. - PubMed
-
- Verri E, Guglielmini P, Puntoni M, et al. HER2/neu oncoprotein overexpression in epithelial ovarian cancer: evaluation of its prevalence and prognostic significance. Clinical study Oncology. 2005;68:154–61. - PubMed
-
- Thor A, Ohuchi N, Szpak CA, Johnston WW, Schlom J. Distribution of oncofetal antigen tumor-associated glycoprotein-72 defined by monoclonal antibody B723. Cancer Res. 1986;46:3118–24. - PubMed
-
- Yin BW, Finstad CL, Kitamura K, et al. Serological and immunochemical analysis of Lewis y (Ley) blood group antigen expression in epithelial ovarian cancer. Int J Cancer. 1996;65:406–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

