The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis
- PMID: 17062726
- PMCID: PMC1794060
- DOI: 10.1182/blood-2006-06-031898
The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis
Abstract
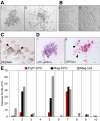
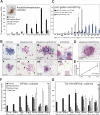
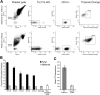
In the adult, platelets are derived from unipotential megakaryocyte colony-forming cells (Meg-CFCs) that arise from bipotential megakaryocyte/erythroid progenitors (MEPs). To better define the developmental origin of the megakaryocyte lineage, several aspects of megakaryopoiesis, including progenitors, maturing megakaryocytes, and circulating platelets, were examined in the murine embryo. We found that a majority of hemangioblast precursors during early gastrulation contains megakaryocyte potential. Combining progenitor assays with immunohistochemical analysis, we identified 2 waves of MEPs in the yolk sac associated with the primitive and definitive erythroid lineages. Primitive MEPs emerge at E7.25 along with megakaryocyte and primitive erythroid progenitors, indicating that primitive hematopoiesis is bilineage in nature. Subsequently, definitive MEPs expand in the yolk sac with Meg-CFCs and definitive erythroid progenitors. The first GP1bbeta-positive cells in the conceptus were identified in the yolk sac at E9.5, while large, highly reticulated platelets were detected in the embryonic bloodstream beginning at E10.5. At this time, the number of megakaryocyte progenitors begins to decline in the yolk sac and expand in the fetal liver. We conclude that the megakaryocyte lineage initially originates from hemangioblast precursors during early gastrulation and is closely associated both with primitive and with definitive erythroid lineages in the yolk sac prior to the transition of hematopoiesis to intraembryonic sites.
Figures



References
-
- Moore MA, Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol. 1970;18:279–296. - PubMed
-
- Sabin FR. Studies on the origin of blood vessels and red blood corpuscles as seen in the living blastoderm of chicks during the second day of incubation. Contrib Embryol. 1920;9:213–262.
-
- Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. - PubMed
-
- Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998;125:1747–1757. - PubMed
-
- Lacaud G, Gore L, Kennedy M, et al. Runx1 is essential for hematopoietic commitment at the hemangioblast stage of development in vitro. Blood. 2002;100:458–466. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

