Structure reassignment and synthesis of Jenamidines A1/A2, synthesis of (+)-NP25302, and formal synthesis of SB-311009 analogues
- PMID: 17064037
- PMCID: PMC2528249
- DOI: 10.1021/jo061650+
Structure reassignment and synthesis of Jenamidines A1/A2, synthesis of (+)-NP25302, and formal synthesis of SB-311009 analogues
Abstract
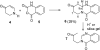
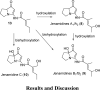
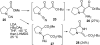
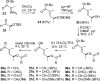
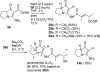
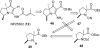
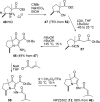
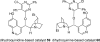
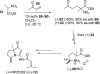
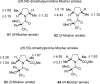
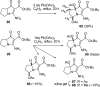
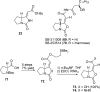
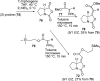
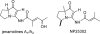
The proposed structures of jenamidines A, B, and C (1-3) were revised to jenamidines A1/A2, B1/B2, and C (8-10). Jenamidines A1/A2 (8) were synthesized from activated proline derivative 43 by conversion to 26 in two steps and 50% overall yield. Acylation of 26 with acid chloride 38d gave 39d, which was deprotected with TFA and then mild base to give 8 in 45% yield from 26. (-)-trans-2,5-Dimethylproline ethyl ester (49) was prepared by the enantioselective Michael reaction of ethyl 2-nitropropionate (51) and methyl vinyl ketone (50) using modified dihydroquinine 60 as the catalyst. Further elaboration converted 49 to natural (+)-NP25302 (12). A Wittig reaction of proline NCA (76) with ylide 79 gave 72 as a 9/1 E/Z mixture in 27% yield, completing a one-step formal synthesis of SB-311009 analogues.
Figures


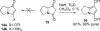





References
-
- Hu J-F, Wunderlich D, Thiericke R, Dahse H-M, Grabley S, Feng X-Z, Sattler I. J. Antibiot. 2003;56:747–754. - PubMed
-
-
For preliminary communication of portions of this work see: Snider BB, Duvall JR, Sattler I, Huang X. Tetrahedron Lett. 2004;45:6725–6727. Snider BB, Duvall JR. Org. Lett. 2005;7:4519–4522.
-
-
- Pouchert CJ, Behnke J. The Aldrich Library of 13C and 1H FT NMR Spectra. Edition 1 Vol. 1. Aldrich; Milwaukee, WI: 1993. p. 1251c.
-
- Raucher S, Macdonald JE. Synth. Commun. 1980;10:325–331.
- Haider A, Cornuz G, Wyler H. Helv. Chim. Acta. 1975;58:1287–1292.
-
-
For the preparation of analogous compounds with the NH replaced by O or S see: Hamley P, Tinker A. Chem. Abstr. 2000;132:137406a. PCT Int. Appl. WO 00 06,576, 2000.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

