Calcitonin gene-related peptide enhances release of native brain-derived neurotrophic factor from trigeminal ganglion neurons
- PMID: 17064360
- PMCID: PMC2440676
- DOI: 10.1111/j.1471-4159.2006.04161.x
Calcitonin gene-related peptide enhances release of native brain-derived neurotrophic factor from trigeminal ganglion neurons
Abstract
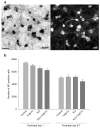
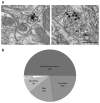
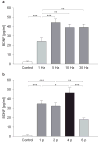
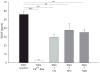
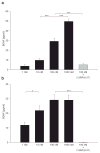
Activity-dependent plasticity in nociceptive pathways has been implicated in pathomechanisms of chronic pain syndromes. Calcitonin gene-related peptide (CGRP), which is expressed by trigeminal nociceptors, has recently been identified as a key player in the mechanism of migraine headaches. Here we show that CGRP is coexpressed with brain-derived neurotrophic factor (BDNF) in a large subset of adult rat trigeminal ganglion neurons in vivo. Using ELISA in situ, we show that CGRP (1-1000 nM) potently enhances BDNF release from cultured trigeminal neurons. The effect of CGRP is dose-dependent and abolished by pretreatment with CGRP receptor antagonist, CGRP(8-37). Intriguingly, CGRP-mediated BDNF release, unlike BDNF release evoked by physiological patterns of electrical stimulation, is independent of extracellular calcium. Depletion of intracellular calcium stores with thapsigargin blocks the CGRP-mediated BDNF release. Using transmission electron microscopy, our study also shows that BDNF-immunoreactivity is present in dense core vesicles of unmyelinated axons and axon terminals in the subnucleus caudalis of the spinal trigeminal nucleus, the primary central target of trigeminal nociceptors. Together, these results reveal a previously unknown role for CGRP in regulating BDNF availability, and point to BDNF as a candidate mediator of trigeminal nociceptive plasticity.
Figures


References
-
- Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, Thadani A, Squinto SP, Yancopoulos GD, Lindsay RM. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. - PubMed
-
- Aicher SA, Mitchell JL, Swanson KC, Zadina JE. Endomorphin-2 axon terminals contact mu-opioid receptor-containing dendrites in trigeminal dorsal horn. Brain Res. 2003;977:190–198. - PubMed
-
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. - PubMed
-
- Akopian AN, Souslova V, England S, et al. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nature Neurosci. 1999;2:541–548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

