Effect of tuberculosis preventive therapy on HIV disease progression and survival in HIV-infected adults
- PMID: 17065029
- PMCID: PMC2860292
- DOI: 10.1310/hct0704-172
Effect of tuberculosis preventive therapy on HIV disease progression and survival in HIV-infected adults
Abstract
Purpose: To determine whether tuberculosis (TB) preventive therapies alter the rate of disease progression to AIDS or death and to identify significant prognostic factors for HIV disease progression to AIDS.
Method: In a randomized placebo-controlled trial in Kampala, Uganda, 2,736 purified protein derivative (PPD)-positive and anergic HIV-infected adults were randomly assigned to four and two regimens, respectively. PPD-positive patients were treated with isoniazid (INH) for 6 months (6H; n = 536), INH plus rifampicin for 3 months (3HR; n = 556), INH plus rifampicin plus pyrazinamide for 3 months (3HRZ; n = 462), or placebo for 6 months (n = 464). Anergic participants were treated with 6H (n = 395) or placebo (n = 323).
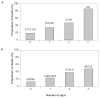
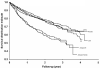
Results: During follow-up, 404 cases progressed to AIDS and 577 deaths occurred. The cumulative incidence of the AIDS progression was greater in the anergic cohort compared to the PPD-positive cohort (p < .0001). Among PPD-positive patients, the relative risk of the AIDS progression with INH alone was 0.95 (95% CI 0.68-1.32); with 3HR it was 0.83 (95% CI 0.59-1.17); and with 3HRZ it was 0.76 (95% CI 0.52-1.08), controlling for significant baseline predictors. Among anergic patients, the relative risk of the AIDS progression was 0.81 (95% CI 0.56-1.15). Survival was greater in the PPD-positive cohort compared to the anergic cohort (p = .0001).
Conclusion: The number of signs or symptoms at baseline and anergic status are associated with increasing morbidity and mortality. Even though the tuberculosis preventive therapies were effective in reducing the incidence of TB for HIV-infected adults, their benefit of delaying HIV disease progression to AIDS was not observed.
Figures
References
-
- Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337:725–733. - PubMed
-
- Gulick RM, Mellors JW, Havlir D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. - PubMed
-
- Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human deficiency virus infection. N Engl J Med. 1998;338:853–856. - PubMed
-
- Hogg RS, O'Shaughnessy MV, Gataric N, et al. Decline in deaths from AIDS due to new antiretrovirals. Lancet. 1997;349:1294. - PubMed
-
- UNAIDS Report on the global HIV/AIDS epidemic, December 2004. Available at: http://www.usaids.org/publications/index.html. Accessed September 1, 2005.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical