Ca2+ sparks and T tubule reorganization in dedifferentiating adult mouse skeletal muscle fibers
- PMID: 17065203
- PMCID: PMC2654399
- DOI: 10.1152/ajpcell.00397.2006
Ca2+ sparks and T tubule reorganization in dedifferentiating adult mouse skeletal muscle fibers
Abstract
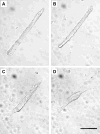
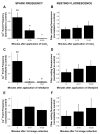
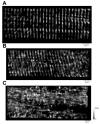
Ca(+) sparks are rare in healthy adult mammalian skeletal muscle but may appear when adult fiber integrity is compromised, and occur in embryonic muscle but decline as the animal develops. Here we used cultured adult mouse flexor digitorum brevis muscle fibers to monitor occurrence of Ca(2+) sparks during maintenance of adult fiber morphology and during eventual fiber morphological dedifferentiation after various times in culture. Fibers cultured for up to 3 days retain normal morphology and striated appearance. Ca(2+) sparks were rare in these fibers. At 5-7 days in culture, many of the original muscle fibers exhibit sprouting and loss of striations, as well as the occurrence of spontaneous Ca(2+) sparks. The average rate of occurrence of Ca(2+) sparks is >10-fold higher after 5-7 days in culture than in days 1-3. With the use of fibers cultured for 7 days, application of the Ca(2+) channel blockers Co(2+) or nifedipine almost completely suppressed the occurrence of Ca(2+) sparks, as previously shown in embryonic fibers, suggesting that Ca(2+) sparks may be generated by similar mechanisms in dedifferentiating cultured adult fibers and in embryonic fibers before final differentiation. The sarcomeric disruption observed under transmitted light microscopy in dedifferentiating fibers was accompanied by morphological changes in the transverse (T) tubular system, as observed by fluorescence confocal imaging of both an extracellular marker dye and membrane staining dyes. Changes in T tubule morphology coincided with the appearance of Ca(2+) sparks, suggesting that Ca(2+) sparks may either be a signal for, or the result of, disruption of DHPR-ryanodine receptor 1 coupling.
Figures







References
-
- Brown LD, Schneider MF. Delayed differentiation and retention of properties in dissociated adult skeletal muscle fibers in vitro. In Vitro Cell Dev Biol-Animal. 2002;38:411–422. - PubMed
-
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

