Mechanisms of gastrointestinal CD4+ T-cell depletion during acute and early human immunodeficiency virus type 1 infection
- PMID: 17065209
- PMCID: PMC1797467
- DOI: 10.1128/JVI.01739-06
Mechanisms of gastrointestinal CD4+ T-cell depletion during acute and early human immunodeficiency virus type 1 infection
Abstract
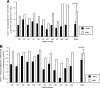
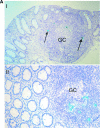
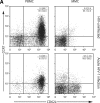
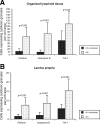
During acute and early human immunodeficiency virus type 1 (HIV-1) infection (AEI) more than 50% of CD4+ T cells are preferentially depleted from the gastrointestinal (GI) lamina propria. To better understand the underlying mechanisms, we studied virological and immunological events within the peripheral blood (PB) and GI tract during AEI. A total of 32 AEI subjects and 18 uninfected controls underwent colonic biopsy. HIV-1 viral DNA and RNA levels were quantified in CD4+ T cells derived from the GI tract and PB by using real-time PCR. The phenotype of infected cells was characterized by using combinations of immunohistochemistry and in situ hybridization. Markers of immunological memory, activation, and proliferation were examined by flow cytometry and immunohistochemistry, and the host-derived cytotoxic cellular response was examined by using immunohistochemistry. GI CD4+ T cells harbored, on average, 13-fold higher HIV-1 viral DNA levels and 10-fold higher HIV-1 RNA levels than PB CD4+ T cells during AEI. HIV-1 RNA was detected in both "activated" and "nonactivated" mucosal CD4+ T cells. A significantly higher number of activated and proliferating T cells were detected in the GI tract compared to the PB, and a robust cytotoxic response (HIV-1 specificity not determined) was detected in the GI tract as early as 18 days postinfection. Mucosal CD4+ T-cell depletion is multifactorial. Direct viral infection likely accounts for the earliest loss of CD4+ T cells. Subsequently, ongoing infection of susceptible CD4+ T cells, along with activation-induced cellular death and host cytotoxic cellular response, are responsible for the persistence of the lesion.
Figures










References
-
- Andersson, J., H. Behbahani, J. Lieberman, E. Connick, A. Landay, B. Patterson, A. Sonnerborg, K. Lore, S. Uccini, and T. E. Fehniger. 1999. Perforin is not coexpressed with granzyme A within cytotoxic granules in CD8 T lymphocytes present in lymphoid tissue during chronic HIV infection. Aids 13:1295-1303. - PubMed
-
- Andersson, J., S. Kinloch, A. Sonnerborg, J. Nilsson, T. E. Fehniger, A. L. Spetz, H. Behbahani, L. E. Goh, H. McDade, B. Gazzard, H. Stellbrink, D. Cooper, and L. Perrin. 2002. Low levels of perforin expression in CD8+ T lymphocyte granules in lymphoid tissue during acute human immunodeficiency virus type 1 infection. J. Infect. Dis. 185:1355-1358. - PubMed
-
- Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T-cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. - PMC - PubMed
-
- Estes, J. D., B. F. Keele, K. Tenner-Racz, P. Racz, M. A. Redd, T. C. Thacker, Y. Jiang, M. J. Lloyd, S. Gartner, and G. F. Burton. 2002. Follicular dendritic cell-mediated up-regulation of CXCR4 expression on CD4 T cells and HIV pathogenesis. J. Immunol. 169:2313-2322. - PubMed
-
- Fackler, O. T., M. Schafer, W. Schmidt, T. Zippel, W. Heise, T. Schneider, M. Zeitz, E. O. Riecken, N. Mueller-Lantzsch, and R. Ullrich. 1998. HIV-1 p24 but not proviral load is increased in the intestinal mucosa compared with the peripheral blood in HIV-infected patients. AIDS 12:139-146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

