A large-conductance calcium-selective mechanotransducer channel in mammalian cochlear hair cells
- PMID: 17065441
- PMCID: PMC6674673
- DOI: 10.1523/JNEUROSCI.2188-06.2006
A large-conductance calcium-selective mechanotransducer channel in mammalian cochlear hair cells
Abstract
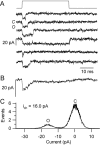
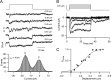
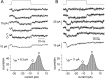
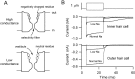
Sound stimuli are detected in the cochlea by opening of hair cell mechanotransducer (MT) channels, one of the few ion channels not yet conclusively identified at a molecular level. To define their performance in situ, we measured MT channel properties in inner hair cells (IHCs) and outer hair cells (OHCs) at two locations in the rat cochlea tuned to different characteristic frequencies (CFs). The conductance (in 0.02 mM calcium) of MT channels from IHCs was estimated as 260 pS at both low-frequency and mid-frequency positions, whereas that from OHCs increased with CFs from 145 to 210 pS. The combination of MT channel conductance and tip link number, assayed from scanning electron micrographs, accounts for variation in whole-cell current amplitude for OHCs and its invariance for IHCs. Channels from apical IHCs and OHCs having a twofold difference in unitary conductance were both highly calcium selective but were distinguishable by a small but significant difference in calcium permeability and in their response to lowering ionic strength. The results imply that the MT channel has properties possessed by few known candidates, and its diversity suggests expression of multiple isoforms.
Figures





References
-
- Assad JA, Shepherd GM, Corey DP. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron. 1991;7:985–994. - PubMed
-
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. - PubMed
-
- Bosher SK, Warren RL. Very low calcium content of cochlear endolymph, an extracellular fluid. Nature. 1978;273:377–378. - PubMed
-
- Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57:427–450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
