Potential new antiepileptogenic targets indicated by microarray analysis in a rat model for temporal lobe epilepsy
- PMID: 17065450
- PMCID: PMC6674659
- DOI: 10.1523/JNEUROSCI.2766-06.2006
Potential new antiepileptogenic targets indicated by microarray analysis in a rat model for temporal lobe epilepsy
Abstract
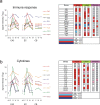
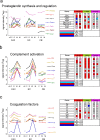
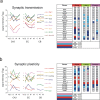
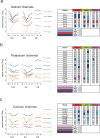
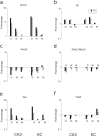
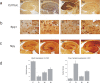
To get insight into the mechanisms that may lead to progression of temporal lobe epilepsy, we investigated gene expression during epileptogenesis in the rat. RNA was obtained from three different brain regions [CA3, entorhinal cortex (EC), and cerebellum (CB)] at three different time points after electrically induced status epilepticus (SE): acute phase [group D (1 d)], latent period [group W (1 week)], and chronic epileptic period [group M (3-4 months)]. A group that was stimulated but that had not experienced SE and later epilepsy was also included (group nS). Gene expression analysis was performed using the Affymetrix Gene Chip System (RAE230A). We used GENMAPP and Gene Ontology to identify global biological trends in gene expression data. The immune response was the most prominent process changed during all three phases of epileptogenesis. Synaptic transmission was a downregulated process during the acute and latent phases. GABA receptor subunits involved in tonic inhibition were persistently downregulated. These changes were observed mostly in both CA3 and EC but not in CB. Rats that were stimulated but that did not develop spontaneous seizures later on had also some changes in gene expression, but this was not reflected in a significant change of a biological process. These data suggest that the targeting of specific genes that are involved in these biological processes may be a promising strategy to slow down or prevent the progression of epilepsy. Especially genes related to the immune response, such as complement factors, interleukins, and genes related to prostaglandin synthesis and coagulation pathway may be interesting targets.
Figures


References
-
- Aloisi F. The role of microglia and astrocytes in CNS immune surveillance and immunopathology. Adv Exp Med Biol. 1999;468:123–133. - PubMed
-
- Arion D, Sabatini M, Unger T, Pastor J, Alonso-Nanclares L, Ballesteros-Yanez I, Garcia Sola R, Munoz A, Mirnics K, Defelipe J. Correlation of transcriptome profile with electrical activity in temporal lobe epilepsy. Neurobiol Dis. 2006;22:374–387. - PubMed
-
- Aronica E, Vliet van EA, Mayboroda O, Troost D, Lopes da Silva FH, Gorter JA. Upregulation of metabotropic glutamate receptor subtype mGluR3 and mGluR5 in reactive astrocytes in a rat model of mesial temporal lobe epilepsy. Eur J Neurosci. 2000;12:2333–2345. - PubMed
-
- Avoli M, D'Antuono M, Louvel J, Kohling R, Biagini G, Pumain R, D'Arcangelo G, Tancredi V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog Neurobiol. 2002;68:167–207. - PubMed
-
- Bammler T, Beyer RP, Bhattacharya S, Boorman GA, Boyles A, Bradford BU, Bumgarner RE, Bushel PR, Chaturvedi K, Choi D, Cunningham ML, Deng S, Dressman HK, Fannin RD, Farin FM, Freedman JH, Fry RC, Harper A, Humble MC, Hurban P, et al. Standardizing global gene expression analysis between laboratories and across platforms. Nat Methods. 2005;2:351–356. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
