The NHR1 domain of Neuralized binds Delta and mediates Delta trafficking and Notch signaling
- PMID: 17065551
- PMCID: PMC1751308
- DOI: 10.1091/mbc.e06-08-0753
The NHR1 domain of Neuralized binds Delta and mediates Delta trafficking and Notch signaling
Abstract
Notch signaling, which is crucial to metazoan development, requires endocytosis of Notch ligands, such as Delta and Serrate. Neuralized is a plasma membrane-associated ubiquitin ligase that is required for neural development and Delta internalization. Neuralized is comprised of three domains that include a C-terminal RING domain and two neuralized homology repeat (NHR) domains. All three domains are conserved between organisms, suggesting that these regions of Neuralized are functionally important. Although the Neuralized RING domain has been shown to be required for Delta ubiquitination, the function of the NHR domains remains elusive. Here we show that neuralized, a well-characterized neurogenic allele, exhibits a mutation in a conserved residue of the NHR1 domain that results in mislocalization of Neuralized and defects in Delta binding and internalization. Furthermore, we describe a novel isoform of Neuralized and show that it is recruited to the plasma membrane by Delta and that this is mediated by the NHR1 domain. Finally, we show that the NHR1 domain of Neuralized is both necessary and sufficient to bind Delta. Altogether, our data demonstrate that NHR domains can function in facilitating protein-protein interactions and in the case of Neuralized, mediate binding to its ubiquitination target, Delta.
Figures

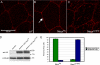

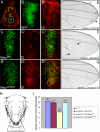

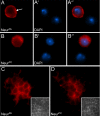
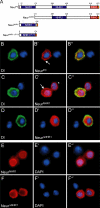
References
-
- Baker N. E. Notch signaling in the nervous system. Pieces still missing from the puzzle. Bioessays. 2000;22:264–273. - PubMed
-
- Bardin A. J., Schweisguth F. Bearded family members inhibit Neuralized-mediated endocytosis and signaling activity of Delta in Drosophila. Dev. Cell. 2006;10:245–255. - PubMed
-
- Brand A. H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
-
- Bray S. Notch signalling in Drosophila: three ways to use a pathway. Semin. Cell Dev. Biol. 1998;9:591–597. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

