Plasma membrane sterol distribution resembles the surface topography of living cells
- PMID: 17065557
- PMCID: PMC1751316
- DOI: 10.1091/mbc.e06-05-0445
Plasma membrane sterol distribution resembles the surface topography of living cells
Abstract
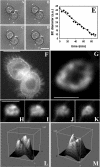
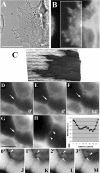
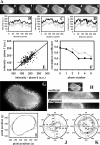
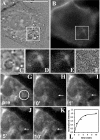
Cholesterol is an important constituent of cellular membranes. It has been suggested that cholesterol segregates into sterol-rich and -poor domains in the plasma membrane, although clear evidence for this is lacking. By fluorescence imaging of the natural sterol dehydroergosterol (DHE), the lateral sterol distribution has been visualized in living cells. The spatial labeling pattern of DHE coincided with surface structures such as ruffles, microvilli, and filopodia with correlation lengths in the range of 0.8-2.5 microm. DHE staining of branched tubules and of nanotubes connecting two cells was detected. Dynamics of DHE in folded and plane membrane regions was comparable as determined by fluorescence recovery after photobleaching. DHE colocalized with fluid membrane-preferring phospholipids in surface structures and at sites of cell attachment as well as in the cleavage furrow of dividing cells, but it was not particularly enriched in those regions. Fluorescent sterol showed homogeneous staining in membrane blebs induced by F-actin disruption. Cross-linking the ganglioside GM1--a putative raft marker--did not affect the cell surface distribution of DHE. The results suggest that spatial heterogeneities of plasma membrane staining of DHE resolvable by light microscopy reflect the cell surface topography but not phase-separated sterol domains in the bilayer plane.
Figures




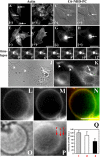
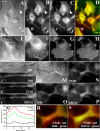
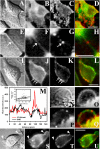
Similar articles
-
Spatiotemporal analysis of endocytosis and membrane distribution of fluorescent sterols in living cells.Histochem Cell Biol. 2008 Nov;130(5):891-908. doi: 10.1007/s00418-008-0488-6. Epub 2008 Sep 12. Histochem Cell Biol. 2008. PMID: 18787836
-
Direct observation of rapid internalization and intracellular transport of sterol by macrophage foam cells.Traffic. 2005 May;6(5):396-412. doi: 10.1111/j.1600-0854.2005.00285.x. Traffic. 2005. PMID: 15813750
-
Potential of ultraviolet wide-field imaging and multiphoton microscopy for analysis of dehydroergosterol in cellular membranes.Microsc Res Tech. 2011 Jan;74(1):92-108. doi: 10.1002/jemt.20878. Microsc Res Tech. 2011. PMID: 21181715
-
Fluorescence techniques using dehydroergosterol to study cholesterol trafficking.Lipids. 2008 Dec;43(12):1185-208. doi: 10.1007/s11745-008-3194-1. Epub 2008 Jun 7. Lipids. 2008. PMID: 18536950 Free PMC article. Review.
-
Fluorescent sterols as tools in membrane biophysics and cell biology.Chem Phys Lipids. 2007 Mar;146(1):1-25. doi: 10.1016/j.chemphyslip.2006.12.004. Epub 2006 Dec 30. Chem Phys Lipids. 2007. PMID: 17241621 Review.
Cited by
-
Time-Resolved Laurdan Fluorescence Reveals Insights into Membrane Viscosity and Hydration Levels.Biophys J. 2018 Oct 16;115(8):1498-1508. doi: 10.1016/j.bpj.2018.08.041. Epub 2018 Sep 6. Biophys J. 2018. PMID: 30269886 Free PMC article.
-
Cholesterol binding to a conserved site modulates the conformation, pharmacology, and transport kinetics of the human serotonin transporter.J Biol Chem. 2018 Mar 9;293(10):3510-3523. doi: 10.1074/jbc.M117.809046. Epub 2018 Jan 19. J Biol Chem. 2018. PMID: 29352106 Free PMC article.
-
Spatiotemporal analysis of endocytosis and membrane distribution of fluorescent sterols in living cells.Histochem Cell Biol. 2008 Nov;130(5):891-908. doi: 10.1007/s00418-008-0488-6. Epub 2008 Sep 12. Histochem Cell Biol. 2008. PMID: 18787836
-
Structure of dehydroergosterol monohydrate and interaction with sterol carrier protein-2.Lipids. 2008 Dec;43(12):1165-84. doi: 10.1007/s11745-008-3267-1. Epub 2008 Nov 20. Lipids. 2008. PMID: 19020914 Free PMC article.
-
Tunneling-nanotube direction determination in neurons and astrocytes.Cell Death Dis. 2012 Dec 6;3(12):e438. doi: 10.1038/cddis.2012.177. Cell Death Dis. 2012. PMID: 23222508 Free PMC article.
References
-
- Ahmed S. N., Brown D. A., London E. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry. 1997;36:10944–10953. - PubMed
-
- Braet F., Seynaeve C., De Zanger R., Wisse E. Imaging surface and submembranous structures with the atomic force microscope: a study on living cancer cells, fibroblasts and macrophages. J. Microsc. 1998;190:328–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

