Population pharmacokinetics of Arbekacin in patients infected with methicillin-resistant Staphylococcus aureus
- PMID: 17065621
- PMCID: PMC1635234
- DOI: 10.1128/AAC.00420-05
Population pharmacokinetics of Arbekacin in patients infected with methicillin-resistant Staphylococcus aureus
Abstract
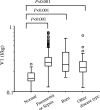
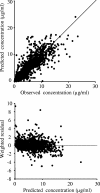
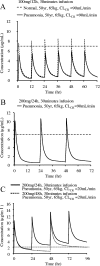
Arbekacin, a derivative of dibekacin, is an aminoglycoside developed and widely used in Japan for the treatment of patients infected with methicillin-resistant Staphylococcus aureus (MRSA). The population pharmacokinetics of arbekacin was investigated in the Japanese, using 353 patients infected with MRSA and 50 healthy or renally impaired volunteers. The age of the study population ranged from 8 to 95 years, and weight ranged from 10.8 to 107 kg. In total, 1,581 serum arbekacin concentrations were measured (primarily from routine patient care) and used to perform the present pharmacokinetic analysis. Drug concentration-time data were well described by a two-compartment open model. Factors influencing arbekacin pharmacokinetics were investigated using a nonlinear mixed-effect model analysis. The best-developed model showed that drug clearance (CL) was related to creatinine clearance (CL(CR)), age, and body weight (WT), as expressed by CL (liter/h) = 0.0319CL(CR) + (26.5/age) (CL(CR) < 80 ml/min) and CL (liter/h) = 0.0130 CL(CR) + 0.0342WT + (26.5/age) (CL(CR) >/= 80 ml/min). The volume of distribution for the central and peripheral compartments was different in healthy subjects and infected patients, and this difference was more pronounced among disease types. The elderly subjects (aged 80 years or over) exhibited, on average, a 19% greater volume for the central compartment. The volumes for the peripheral compartment were 50.6 liters in patients with pneumonia and 24.3 liters in patients with sepsis. The population pharmacokinetic parameters of arbekacin obtained here are useful for optimal use of this aminoglycoside in the treatment of MRSA-infected patients.
Figures


References
-
- Aoki, Y. 1994. Bactericidal activity of arbekacin against methicillin-resistant Staphylococcus aureus. Comparison with that of vancomycin. Jpn. J. Antibiot. 47:640-646. - PubMed
-
- Arakawa, S., H. Maeda, A. Fujii, S. Kamidono, K. Hamada, S. Miyazaki, and S. Hara. 1989. Clinical study of aminoglycoside on renal dysfunction. Pharmacokinetics of arbekacin and its elimination effects by hemodialysis and adsorption with charcoal. Acta Urol. Jpn. 35:697-704. - PubMed
-
- Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. - PubMed
-
- de Hoog, M., R. C. Schoemaker, J. W. Mouton, and J. N. van den Anker. 1997. Tobramycin population pharmacokinetics in neonates. Clin. Pharmacol. Ther. 62:392-399. - PubMed
-
- Ette, E. I. 1997. Stability and performance of a population pharmacokinetic model. J. Clin. Pharmacol. 37:486-495. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

