Studies on the mode of action of the antifungal hexapeptide PAF26
- PMID: 17065623
- PMCID: PMC1635192
- DOI: 10.1128/AAC.00650-06
Studies on the mode of action of the antifungal hexapeptide PAF26
Abstract
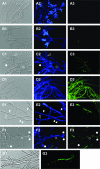
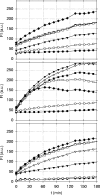
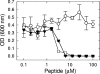
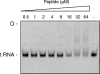
The small antimicrobial peptide PAF26 (Ac-RKKWFW-NH(2)) has been identified by a combinatorial approach and shows preferential activity toward filamentous fungi. In this work, we investigated the mode of action and inhibitory effects of PAF26 on the fungus Penicillium digitatum. The dye Sytox Green was used to demonstrate that PAF26 induced cell permeation. However, microscopic observations showed that sub-MIC concentrations of PAF26 produced both alterations of hyphal morphology (such as altered polar growth and branching) and chitin deposition in areas of no detectable permeation. Analysis of dose-response curves of inhibition and permeation suggested that growth inhibition is not solely a consequence of permeation. In order to shed light on the mode of PAF26 action, its antifungal properties were compared with those of melittin, a well-known pore-forming peptide that kills through cytolysis. While the 50% inhibitory concentrations and MICs of the two peptides against P. digitatum mycelium were comparable, they differed markedly in their fungicidal activities toward conidia and their hemolytic activities toward human red blood cells. Kinetic studies showed that melittin quickly induced Penicillium cell permeation, while PAF26-induced Sytox Green uptake was significantly slower and less efficient. Therefore, the ultimate growth inhibition and morphological alterations induced by PAF26 for P. digitatum are not likely a result of conventional pore formation. Fluorescently labeled PAF26 was used to demonstrate its specific in vivo interaction and translocation inside germ tubes and hyphal cells, at concentrations as low as 0.3 muM (20 times below the MIC), at which no inhibitory, morphological, or permeation effects were observed. Interestingly, internalized PAF26 could bind to cellular RNAs, since in vitro nonspecific RNA binding activity of PAF26 was demonstrated by electrophoretic mobility shift assays. We propose that PAF26 is a short, de novo-designed penetratin-type peptide that has multiple detrimental effects on target fungi, which ultimately result in permeation and killing.
Figures



References
-
- Broekaert, W. F., F. R. G. Terras, B. P. A. Cammue, and J. Vanderleyden. 1990. An automated quantitative assay for fungal growth-inhibition. FEMS Microbiol. Lett. 69:55-59.
-
- Brogden, K. A. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3:238-250. - PubMed
-
- Epand, R. M., and H. J. Vogel. 1999. Diversity of antimicrobial peptides and their mechanisms of action. Biochim. Biophys. Acta 1462:11-28. - PubMed
-
- Gonzalez, C. F., E. M. Provin, L. Zhu, and D. J. Ebbole. 2002. Independent and synergistic activity of synthetic peptides against thiabendazole-resistant Fusarium sambucinum. Phytopathology 92:917-924. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

