Glucocorticoid-induced tumour necrosis factor receptor family related protein (GITR) mediates inflammatory activation of macrophages that can destabilize atherosclerotic plaques
- PMID: 17067317
- PMCID: PMC1819571
- DOI: 10.1111/j.1365-2567.2006.02453.x
Glucocorticoid-induced tumour necrosis factor receptor family related protein (GITR) mediates inflammatory activation of macrophages that can destabilize atherosclerotic plaques
Abstract
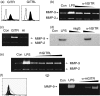
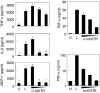
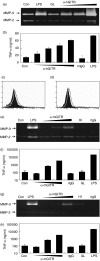
Glucocorticoid-induced tumour necrosis factor receptor family related protein (GITR) is the 18th member of the tumour necrosis factor receptor superfamily (TNFRSF18) and is known to interact with its cognate ligand GITRL (TNFSF18). We investigated the potential role of GITR in the pro-inflammatory activation of macrophages. Immunohistochemistry and in situ hybridization analyses of human atherosclerotic plaques demonstrated that GITR and its ligand are expressed mainly in lipid-rich macrophages. We then investigated the role of GITR in human and mouse monocyte/macrophage functions. Stimulation of GITR caused nuclear factor (NF)-kappaB-dependent activation of matrix metalloproteinase-9 (MMP-9) and pro-inflammatory cytokine expression in both the human and mouse monocytic/macrophage cell lines, THP-1 and RAW264.7, respectively. These cellular responses were also observed when the THP-1 cells were treated with phorbol-12 myristate-13 acetate (PMA), which is known to induce macrophage differentiation. To demonstrate that these responses are not restricted to cultured cell lines, we tested primary macrophages. Both peritoneal and bone marrow-derived macrophages responded to GITR stimulation with induction of MMP-9 and tumour necrosis factor-alpha (TNF-alpha). Furthermore, the GITR staining pattern overlapped with those of MMP-9 and TNF-alpha in atherosclerotic plaques. These data indicate that GITR-mediated macrophage activation may promote atherogenesis via the induction of pro-atherogenic cytokines/chemokines, and destabilize the atherosclerotic plaques via the induction of the matrix-degrading enzyme, MMP-9.
Figures



References
-
- Rus HG, Niculescu F, Vlaicu R. Tumor necrosis factor-alpha in human arterial wall with atherosclerosis. Atherosclerosis. 1991;89:247–54. - PubMed
-
- Kaartinen M, Penttila A, Kovanen PT. Mast cells in rupture-prone areas of human coronary atheromas produce and store TNF-α. Circulation. 1996;94:2787–92. - PubMed
-
- Mach F, Schonbeck U, Sukhova GK, Bourcier T, Bonnefoy JY, Pober JS, Libby P. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40–CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci USA. 1997;94:1931–6. - PMC - PubMed
-
- Lee WH, Kim SH, Lee Y, Lee BB, Kwon B, Song H, Kwon BS, Park JE. Tumor necrosis factor receptor superfamily 14 is involved in atherogenesis by inducing proinflammatory cytokines and matrix metalloproteinases. Arterioscler Thromb Vasc Biol. 2001;21:2004–10. - PubMed
-
- Kim WJ, Lee WH. LIGHT is expressed in foam cells and involved in destabilization of atherosclerotic plaques through induction of matrix metalloproteinase-9 and IL-8. Immune Network. 2004;4:116–22.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

