Mechanism of glucocorticoid regulation of the intestinal tight junction barrier
- PMID: 17068119
- PMCID: PMC3724219
- DOI: 10.1152/ajpgi.00252.2006
Mechanism of glucocorticoid regulation of the intestinal tight junction barrier
Abstract
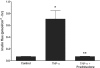
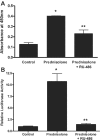
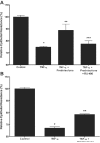
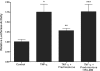
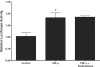
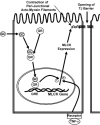
A defective intestinal epithelial tight junction (TJ) barrier has been proposed as an important pathogenic factor contributing to the intestinal inflammation of Crohn's disease. Glucocorticoids are first-line therapeutic agents for the treatment of moderate to severe Crohn's disease. Glucocorticoid treatment has been shown to induce retightening of the intestinal TJ barrier defect in Crohn's disease patients. However, the mechanisms that mediate the glucocorticoid therapeutic action on intestinal TJ barrier function remain unknown. The aim of this study was to elucidate the mechanism of glucocorticoid modulation of the intestinal epithelial TJ barrier using an in vitro model system. Filter-grown Caco-2 intestinal epithelial cells were used as an in vitro model to examine the effects of glucocorticoids on basal intestinal epithelial TJ barrier function and on TNF-alpha-induced disruption of the TJ barrier. Glucocorticoids (prednisolone and dexamethasone) did not have a significant effect on baseline Caco-2 TJ barrier function but prevented the TNF-alpha-induced increase in Caco-2 TJ permeability. The glucocorticoid protective effect against the TNF-alpha-induced increase in Caco-2 TJ permeability required activation of the glucocorticoid receptor (GR) complex. The activation of the GR complex resulted in GR complex binding to the glucocorticoid response element (GRE) site on DNA and activation of a GR-responsive promoter. Glucocorticoids inhibited the TNF-alpha-induced increase in myosin light chain kinase (MLCK) protein expression, a key process mediating the TNF-alpha increase in intestinal TJ permeability. The glucocorticoid inhibition of the TNF-alpha-induced increase in MLCK protein expression was due to the binding of the GR complex to a GRE binding site on the MLCK promoter region suppressing the TNF-alpha-induced activation. Glucocorticoids inhibit the TNF-alpha-induced increase in Caco-2 TJ permeability. The prednisolone protective action was mediated by binding of activated GR complex to the GRE site on the MLCK promoter, suppressing the TNF-alpha-induced increase in MLCK gene activity, protein expression, and subsequent opening of the intestinal TJ barrier.
Figures

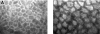

References
-
- Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol Gastrointest Liver Physiol. 1995;269:G467–G475. - PubMed
-
- Arnott ID, Kingstone K, Ghosh S. Abnormal intestinal permeability predicts relapse in inactive Crohn disease. Scand J Gastroenterol. 2000;35:1163–1169. - PubMed
-
- Blair SA, Kane SV, Clayburgh DR, Turner JR. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest. 2006;86:191–201. - PubMed
-
- Fanning AS, Ma TY, Anderson JM. Isolation and functional characterization of the actin binding region in the tight junction protein ZO-1. FASEB J. 2002;16:1835–1837. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

