Structure-guided mutational analysis of T4 RNA ligase 1
- PMID: 17068206
- PMCID: PMC1664725
- DOI: 10.1261/rna.271706
Structure-guided mutational analysis of T4 RNA ligase 1
Abstract
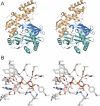
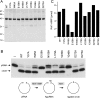
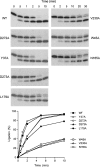
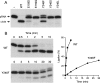
T4 RNA ligase 1 (Rnl1) is a tRNA repair enzyme that circumvents an RNA-damaging host antiviral response. Whereas the three-step reaction scheme of Rnl1 is well established, the structural basis for catalysis has only recently been appreciated as mutational and crystallographic approaches have converged. Here we performed a structure-guided alanine scan of nine conserved residues, including side chains that either contact the ATP substrate via adenine (Leu179, Val230), the 2'-OH (Glu159), or the gamma phosphate (Tyr37) or coordinate divalent metal ions at the ATP alpha phosphate (Glu159, Tyr246) or beta phosphate (Asp272, Asp273). We thereby identified Glu159 and Tyr246 as essential for RNA sealing activity in vitro and for tRNA repair in vivo. Structure-activity relationships at Glu159 and Tyr246 were clarified by conservative substitutions. Eliminating the phosphate-binding Tyr37, and the magnesium-binding Asp272 and Asp273 side chains had little impact on sealing activity in vitro or in vivo, signifying that not all atomic interactions in the active site are critical for function. Analysis of mutational effects on individual steps of the ligation pathway underscored how different functional groups come into play during the ligase-adenylylation reaction versus the subsequent steps of RNA-adenylylation and phosphodiester formation. Moreover, the requirements for sealing exogenous preformed RNA-adenylate are more stringent than are those for sealing the RNA-adenylate intermediate formed in situ during ligation of a 5'-PO4 RNA.
Figures


Similar articles
-
The C-terminal domain of T4 RNA ligase 1 confers specificity for tRNA repair.RNA. 2007 Aug;13(8):1235-44. doi: 10.1261/rna.591807. Epub 2007 Jun 21. RNA. 2007. PMID: 17585047 Free PMC article.
-
Mutational analysis of bacteriophage T4 RNA ligase 1. Different functional groups are required for the nucleotidyl transfer and phosphodiester bond formation steps of the ligation reaction.J Biol Chem. 2003 Aug 8;278(32):29454-62. doi: 10.1074/jbc.M304320200. Epub 2003 May 24. J Biol Chem. 2003. PMID: 12766156
-
Structure-function analysis of the kinase-CPD domain of yeast tRNA ligase (Trl1) and requirements for complementation of tRNA splicing by a plant Trl1 homolog.Nucleic Acids Res. 2006 Jan 20;34(2):517-27. doi: 10.1093/nar/gkj441. Print 2006. Nucleic Acids Res. 2006. PMID: 16428247 Free PMC article.
-
RNA ligase; picking up the pieces.Mol Cell. 2004 Feb 27;13(4):455-6. doi: 10.1016/s1097-2765(04)00089-9. Mol Cell. 2004. PMID: 14992715 Review.
-
Insights into the structure and function of the RNA ligase RtcB.Cell Mol Life Sci. 2023 Nov 7;80(12):352. doi: 10.1007/s00018-023-05001-5. Cell Mol Life Sci. 2023. PMID: 37935993 Free PMC article. Review.
Cited by
-
Caveat mutator: alanine substitutions for conserved amino acids in RNA ligase elicit unexpected rearrangements of the active site for lysine adenylylation.Nucleic Acids Res. 2020 Jun 4;48(10):5603-5615. doi: 10.1093/nar/gkaa238. Nucleic Acids Res. 2020. PMID: 32315072 Free PMC article.
-
PAR-dCLIP: Enabling detection of RNA binding protein target transcripts bound at 5' termini through the incorporation of a decapping step.Methods Enzymol. 2024;705:159-222. doi: 10.1016/bs.mie.2024.08.003. Epub 2024 Sep 7. Methods Enzymol. 2024. PMID: 39389663 Free PMC article.
-
Viroid RNA redirects host DNA ligase 1 to act as an RNA ligase.Proc Natl Acad Sci U S A. 2012 Aug 21;109(34):13805-10. doi: 10.1073/pnas.1206187109. Epub 2012 Aug 6. Proc Natl Acad Sci U S A. 2012. PMID: 22869737 Free PMC article.
-
RNA repair: an antidote to cytotoxic eukaryal RNA damage.Mol Cell. 2008 Jul 25;31(2):278-86. doi: 10.1016/j.molcel.2008.05.019. Mol Cell. 2008. PMID: 18657509 Free PMC article.
-
The C-terminal domain of T4 RNA ligase 1 confers specificity for tRNA repair.RNA. 2007 Aug;13(8):1235-44. doi: 10.1261/rna.591807. Epub 2007 Jun 21. RNA. 2007. PMID: 17585047 Free PMC article.
References
-
- Blondal, T., Thorisdottir, A., Unnsteinsdottir, U., Hjorleifsdottir, S., Aevarsson, A., Ernstsson, S., Fridjonsson, O.H., Skirnisdottir, S., Wheat, J.O., Hermannsdottir, A.G., et al. Isolation and characterization of a thermostable RNA ligase 1 from a Thermus scotoductus bacteriophage TS2126 with good single-stranded DNA ligation properties. Nucleic Acids Res. 2005;33:135–142. - PMC - PubMed
-
- Cranston, J.W., Silber, R., Malathi, V.G., Hurwitz, J. Studies on ribonucleic acid ligase: Characterization of an adenosine triphosphate-inorganic pyrophosphate exchange reaction and demonstration of an enzyme-adenylate complex with T4 bacteriophage-induced enzyme. J. Biol. Chem. 1974;249:7447–7456. - PubMed
-
- Deng, J., Schnaufer, A., Salavati, R., Stuart, K.D., Hol, W.G.J. High resolution crystal structure of a key editosome enzyme from Trypanosoma brucei: RNA editing ligase 1. J. Mol. Biol. 2004;343:601–613. - PubMed
-
- El Omari, K., Ren, J., Bird, L.E., Bona, M.K., Klarmann, G., LeGrice, S.F.J., Stammers, D.K. Molecular architecture and ligand recognition determinants for T4 RNA ligase. J. Biol. Chem. 2006;281:1573–1579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
