Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through Rho/PI 3-kinase
- PMID: 17070513
- PMCID: PMC1913192
- DOI: 10.1016/j.ydbio.2006.09.048
Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through Rho/PI 3-kinase
Abstract
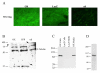
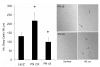
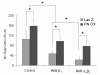
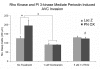
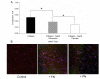
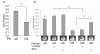
Recent evidence suggests that extracellular matrix components may play a signaling role in embryonic valve development. We have previously identified the spatiotemporal expression patterns of periostin in developing valves, but its function during this process is largely unknown. To evaluate the functional role periostin plays during valvulogenesis, two separate three-dimensional culture assay systems, which model chick atrioventricular cushion development, were employed. These assays demonstrated that cushion mesenchymal cells adhered and spread on purified periostin in a dose-responsive manner, similar to collagen I and fibronectin via alpha(v)beta(3) and beta(1) integrin pairs. Periostin overexpression resulted in enhanced mesenchyme invasion through 3D collagen gels and increased matrix compaction. This invasion was dependent on alpha(v)beta(3) more than beta(1) integrin signaling, and was mediated differentially by Rho kinase and PI 3-kinase. Both matrix invasion and compaction were associated with a colocalization of periostin and beta(1) integrin expression to migratory cell phenotype in both surface and deep cells. The Rho/PI 3-kinase pathway also differentially mediated matrix compaction. Both Rho and PI 3-kinase were involved in normal cushion mesenchyme matrix compaction, but only PI 3-kinase was required for the enhanced matrix compaction due to periostin. Taken together, these results highlight periostin as a mediator of matrix remodeling by cushion mesenchyme towards a mature valve structure.
Figures

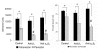


References
-
- Baldwin HS, Lloyd TR, Solursh M. Hyaluronate degradation affects ventricular function of the early postlooped embryonic rat heart in situ. Circ Res. 1994;74:244–52. - PubMed
-
- Banyard J, Anand-Apte B, Symons M, Zetter BR. Motility and invasion are differentially modulated by Rho family GTPases. Oncogene. 2000;19:580–91. - PubMed
-
- Borck A, Massey E, Loftis MJ, Carver W. Exposure of cardiac fibroblasts to the herbicide nitrofen causes altered interactions with the extracellular matrix. Cell Biol Toxicol. 2004;20:15–24. - PubMed
-
- Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608–16. - PubMed
-
- Butcher JT, Penrod AM, Garcia AJ, Nerem RM. Unique morphology and focal adhesion development of valvular endothelial cells in static and fluid flow environments. Arterioscler Thromb Vasc Biol. 2004;24:1429–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

