Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer's disease
- PMID: 17070803
- PMCID: PMC1853320
- DOI: 10.1016/j.expneurol.2006.09.018
Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer's disease
Abstract
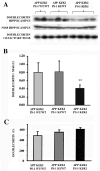
Neurogenesis in the adult hippocampus has been implicated in regulating long-term memory and mood, but its integrity in Alzheimer's disease (AD) is uncertain. Studies of neurogenesis in transgenic mouse models of familial AD are complicated by ectopic overexpression restricted to terminally differentiated neurons, while AD cases have been studied only at the pre-senile or end-stage of disease. To investigate further the fidelity of adult neurogenesis, we examined mice carrying targeted mutations in amyloid precursor protein (APP), presenilin-1 (PS-1), or both APP and PS-1, in which FAD-causing mutations have been inserted into their endogenous genes. The latter "double knock-in" mice developed aging- and region-dependent amyloid deposition starting around 6 months, and by 9 months exhibited microglial activation associated with the amyloid. In the 9-month-old dentate gyrus, the double knock-in mutations reduced the numbers of MCM2-positive neural stem and progenitor cells by 3-fold and doublecortin-positive neuroblasts by 2-fold. The reduction in dentate neuroblasts persisted at 18 months of age. The impairment in neurogenesis was confirmed by quantitative Western blot analysis of doublecortin content and was restricted to the hippocampal but not the olfactory bulb neurogenic system. In contrast, neither mutant PS-1 nor APP alone led to amyloid deposition or significant alterations in the two markers. These results demonstrate long-lasting and selective impairment in adult hippocampal neurogenesis in a knock-in mutant mouse model of FAD and suggest a novel mechanism by which amyloid and its attendant microglia-mediated neuroinflammation could contribute to the cognitive and behavioral abnormalities of AD.
Figures



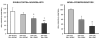

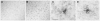
References
-
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. - PubMed
-
- Ashe KH. Learning and memory in transgenic mice modeling Alzheimer's disease. Learn Mem. 2001;8:301–308. - PubMed
-
- Braak H, de Vos RA, Jansen EN, Bratzke H, Braak E. Neuropathological hallmarks of Alzheimer's and Parkinson's diseases. Prog Brain Res. 1998;117:267–285. - PubMed
-
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

