Preferential attachment of peritoneal tumor metastases to omental immune aggregates and possible role of a unique vascular microenvironment in metastatic survival and growth
- PMID: 17071597
- PMCID: PMC1780209
- DOI: 10.2353/ajpath.2006.051222
Preferential attachment of peritoneal tumor metastases to omental immune aggregates and possible role of a unique vascular microenvironment in metastatic survival and growth
Abstract
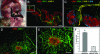
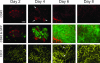
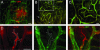
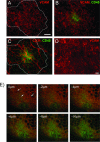
Controlling metastases remains a critical problem in cancer biology. Within the peritoneal cavity, omental tissue is a common site for metastatic disease arising from intraperitoneal tumors; however, it is unknown why this tissue is so favorable for metastatic tumor growth. Using five different tumor cell lines in three different strains of mice, we found that the omentum was a major site of metastases growth for intraperitoneal tumors. Furthermore, initial attachment and subsequent growth were limited to specific sites within the omentum, consisting of organized aggregates of immune cells. These immune aggregates contained a complex network of capillaries exhibiting a high vascular density, which appear to contribute to the survival of metastatic cells. We found that the vasculature within these aggregates contained CD105+ vessels and vascular sprouts, both indicators of active angiogenesis. A subset of mesothelial cells situated atop the immune aggregates was found to be hypoxic, and a similar proportion was observed to secrete vascular endothelial growth factor-A. These data provide a physiological mechanism by which metastatic tumor cells preferentially grow at sites rich in proangiogenic vessels, apparently stimulated by angiogenic factors produced by mesothelial cells. These sites provide metastatic cells with a microenvironment highly conducive to survival and subsequent growth.
Figures




References
-
- Healy JC, Reznek RH. The peritoneum, mesenteries and omenta: normal anatomy and pathological processes. Eur Radiol. 1998;8:886–900. - PubMed
-
- Buy JN, Moss AA, Ghossain MA, Sciot C, Malbec L, Vadrot D, Paniel BJ, Decroix Y. Peritoneal implants from ovarian tumors: CT findings. Radiology. 1988;169:691–694. - PubMed
-
- Sheth S, Horton KM, Garland MR, Fishman EK. Mesenteric neoplasms: CT appearances of primary and secondary tumors and differential diagnosis. Radiographics. 2003;23:435–473. - PubMed
-
- Lawrance RJ, Loizidou M, Cooper AJ, Alexander P, Taylor I. Importance of the omentum in the development of intra-abdominal metastases. Br J Surg. 1991;78:117–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

