Invasive trophoblasts stimulate vascular smooth muscle cell apoptosis by a fas ligand-dependent mechanism
- PMID: 17071607
- PMCID: PMC1780207
- DOI: 10.2353/ajpath.2006.060265
Invasive trophoblasts stimulate vascular smooth muscle cell apoptosis by a fas ligand-dependent mechanism
Abstract
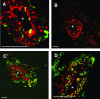
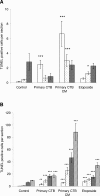
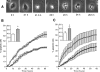
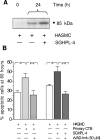
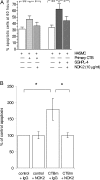
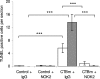
During pregnancy, trophoblasts migrate from the placenta into uterine spiral arteries, transforming them into wide channels that lack vasoconstrictive properties. In pathological pregnancies, this process is incomplete. To define the fundamental events involved in spiral artery remodeling, we have studied the effect of trophoblasts on vascular smooth muscle cells (SMCs). Here we demonstrate for the first time that apoptosis of SMCs can be initiated by invading trophoblasts. When trophoblasts isolated from normal placenta (primary trophoblasts) or conditioned medium was perfused into spiral or umbilical artery segments, apoptosis of SMCs resulted. Culture of human aortic SMCs (HASMCs) with primary trophoblasts, primary trophoblast-conditioned medium, or a trophoblast-derived cell line (SGHPL-4) also significantly increased SMC apoptosis. Fas is expressed by spiral artery SMCs, and a Fas-activating antibody triggered HASMC apoptosis. Furthermore, a Fas ligand (FasL)-blocking antibody significantly inhibited HASMC apoptosis induced by primary trophoblasts, SGHPL-4, or trophoblast-conditioned medium. Depleting primary trophoblast-conditioned medium of FasL also abrogated SMC apoptosis in vessels in situ. These results suggest that apoptosis triggered by the release of soluble FasL from invading trophoblasts contributes to the loss of smooth muscle from the walls of spiral arteries during pregnancy.
Figures

References
-
- Benirschke K, Kaufmann P. New York: Springer,; Pathology of the Human Placenta. 2000:pp 217–226.
-
- Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. - PubMed
-
- Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, Vercruysse L, van Assche A. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol. 1991;98:648–655. - PubMed
-
- Kadyrov M, Schmitz C, Black S, Kaufmann P, Huppertz B. Pre-eclampsia and maternal anaemia display reduced apoptosis and opposite invasive phenotypes of extravillous trophoblast. Placenta. 2003;24:540–548. - PubMed
-
- Naicker T, Khedun SM, Moodley J, Pijnenborg R. Quantitative analysis of trophoblast invasion in preeclampsia. Acta Obstet Gynecol Scand. 2003;82:722–729. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

