Dissecting the pretransitional conformational changes in aminoacylase I thermal denaturation
- PMID: 17071653
- PMCID: PMC1751394
- DOI: 10.1529/biophysj.106.093666
Dissecting the pretransitional conformational changes in aminoacylase I thermal denaturation
Abstract
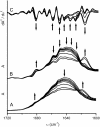
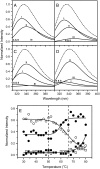
Aminoacylase I (ACYI) catalyzes the stereospecific hydrolysis of L-acylamino acids and is generally assumed to be involved in the final step of the degradation of intracellular N-acetylated proteins. Apart from its crucial functions in intracellular amino acid metabolism, ACYI also has substantial commercial importance for the optical resolution of N-acylated DL-amino acids. As a zinc-dependent enzyme, ACYI is quite stable against heat-induced denaturation and can be regarded as a thermostable enzyme with an optimal temperature for activity of approximately 65 degrees C. In this research, the sequential events in ACYI thermal denaturation were investigated by a combination of spectroscopic methods and related resolution-enhancing techniques. Interestingly, the results from fluorescence and infrared (IR) spectroscopy clearly indicated that a pretransitional stage existed at temperatures from 50 degrees C to 66 degrees C. The thermal unfolding of ACYI might be a three-state process involving an aggregation-prone intermediate appearing at approximately 68 degrees C. The pretransitional structural changes involved the partial unfolding of the solvent-exposed beta-sheet structures and the transformation of about half of the Class I Trp fluorophores to Class II. Our results also suggested that the usage of resolution-enhancing techniques could provide valuable information of the step-wise unfolding of proteins.
Figures






References
-
- Anders, M. W., and W. Dekant. 1994. Aminoacylases. Adv. Pharmacol. 27:431–448. - PubMed
-
- Hernick, M., and C. A. Fierke. 2005. Zinc hydrolases: the mechanisms of zinc-dependent deacetylases. Arch. Biochem. Biophys. 433:71–84. - PubMed
-
- Seibert, C. M., and F. M. Raushel. 2005. Structural and catalytic diversity within the amidohydrolase superfamily. Biochemistry. 44:6383–6391. - PubMed
-
- Birnbaum, S. M. 1955. Aminoacylase, amino acid acylase I and II from hog kidney. Methods Enzymol. 2:115–119.
-
- Kordel, W., and F. Schneider. 1976. Chemical investigation on pig kidney aminoacylase. Biochim. Biophys. Acta. 445:446–457. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

