Identification of a starter unit acyl-carrier protein transacylase domain in an iterative type I polyketide synthase
- PMID: 17071746
- PMCID: PMC1636523
- DOI: 10.1073/pnas.0604112103
Identification of a starter unit acyl-carrier protein transacylase domain in an iterative type I polyketide synthase
Abstract
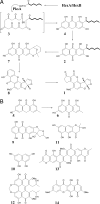
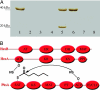
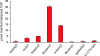
Polyketides are a class of natural products that exhibit a wide range of functional and structural diversity. They include antibiotics, immunosuppressants, antifungals, antihypercholesterolemics, and cytotoxins. Polyketide synthases (PKSs) use chemistry similar to fatty acid synthases (FASs), although building block variation and differing extents of reduction of the growing polyketide chain underlie their biosynthetic versatility. In contrast to the well studied sequential modular type I PKSs, less is known about how the iterative type I PKSs carry out and control chain initiation, elongation, folding, and cyclization during polyketide processing. Domain structure analysis of a group of related fungal, nonreducing PKSs has revealed well defined N-terminal domains longer than commonly seen for FASs and modular PKSs. Predicted structure of this domain disclosed a region similar to malonyl-CoA:acyl-carrier protein (ACP) transacylases (MATs). MATs play a key role transferring precursor CoA thioesters from solution onto FASs and PKSs for chain elongation. On the basis of site-directed mutagenesis, radiolabeling, and kinetics experiments carried out with individual domains of the norsolorinic acid PKS, we propose that the N-terminal domain is a starter unit:ACP transacylase (SAT domain) that selects a C(6) fatty acid from a dedicated yeast-like FAS and transfers this unit onto the PKS ACP, leading to the production of the aflatoxin precursor, norsolorinic acid. These findings could indicate a much broader role for SAT domains in starter unit selection among nonreducing iterative, fungal PKSs, and they provide a biochemical rationale for the classical acetyl "starter unit effect."
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Starter unit flexibility for engineered product synthesis by the nonreducing polyketide synthase PksA.ACS Chem Biol. 2015 Jun 19;10(6):1443-9. doi: 10.1021/acschembio.5b00005. Epub 2015 Mar 10. ACS Chem Biol. 2015. PMID: 25714897 Free PMC article.
-
Ketosynthases in the initiation and elongation modules of aromatic polyketide synthases have orthogonal acyl carrier protein specificity.Biochemistry. 2003 Jun 3;42(21):6588-95. doi: 10.1021/bi0341962. Biochemistry. 2003. PMID: 12767243
-
Characterization of the enzymatic domains in the modular polyketide synthase involved in rifamycin B biosynthesis by Amycolatopsis mediterranei.Gene. 1998 Aug 31;216(2):255-65. doi: 10.1016/s0378-1119(98)00338-2. Gene. 1998. PMID: 9729415
-
The Structural Enzymology of Iterative Aromatic Polyketide Synthases: A Critical Comparison with Fatty Acid Synthases.Annu Rev Biochem. 2018 Jun 20;87:503-531. doi: 10.1146/annurev-biochem-063011-164509. Annu Rev Biochem. 2018. PMID: 29925265 Review.
-
The architectures of iterative type I PKS and FAS.Nat Prod Rep. 2018 Oct 17;35(10):1046-1069. doi: 10.1039/c8np00039e. Nat Prod Rep. 2018. PMID: 30137093 Free PMC article. Review.
Cited by
-
Intrinsic and Extrinsic Programming of Product Chain Length and Release Mode in Fungal Collaborating Iterative Polyketide Synthases.J Am Chem Soc. 2020 Oct 7;142(40):17093-17104. doi: 10.1021/jacs.0c07050. Epub 2020 Sep 23. J Am Chem Soc. 2020. PMID: 32833442 Free PMC article.
-
Bioinformatical analysis of the sequences, structures and functions of fungal polyketide synthase product template domains.Sci Rep. 2015 May 21;5:10463. doi: 10.1038/srep10463. Sci Rep. 2015. PMID: 25995122 Free PMC article.
-
Secondary metabolite profiling, growth profiles and other tools for species recognition and important Aspergillus mycotoxins.Stud Mycol. 2007;59:31-7. doi: 10.3114/sim.2007.59.04. Stud Mycol. 2007. PMID: 18490955 Free PMC article.
-
Recent advancements in the biosynthetic mechanisms for polyketide-derived mycotoxins.Biopolymers. 2010 Sep;93(9):764-76. doi: 10.1002/bip.21483. Biopolymers. 2010. PMID: 20578001 Free PMC article. Review.
-
Deciphering chemical logic of fungal natural product biosynthesis through heterologous expression and genome mining.Nat Prod Rep. 2023 Jan 25;40(1):89-127. doi: 10.1039/d2np00050d. Nat Prod Rep. 2023. PMID: 36125308 Free PMC article. Review.
References
-
- Cortes J, Haydock SF, Roberts GA, Bevitt DJ, Leadlay PF. Nature. 1990;348:176–178. - PubMed
-
- Donadio S, Staver MJ, McAlpine JB, Swanson SJ, Katz L. Science. 1991;252:675–679. - PubMed
-
- Kim ES, Bibb MJ, Butler MJ, Hopwood DA, Sherman DH. Gene. 1994;141:141–142. - PubMed
-
- Staunton J, Wilkinson B. Chem Rev. 1997;97:2611–2630. - PubMed
-
- Hutchinson CR. Chem Rev. 1997;97:2525–2536. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

