Novel acetone metabolism in a propane-utilizing bacterium, Gordonia sp. strain TY-5
- PMID: 17071761
- PMCID: PMC1797311
- DOI: 10.1128/JB.01054-06
Novel acetone metabolism in a propane-utilizing bacterium, Gordonia sp. strain TY-5
Abstract
In the propane-utilizing bacterium Gordonia sp. strain TY-5, propane was shown to be oxidized to 2-propanol and then further oxidized to acetone. In this study, the subsequent metabolism of acetone was studied. Acetone-induced proteins were found in extracts of cells induced by acetone, and a gene cluster designated acmAB was cloned on the basis of the N-terminal amino acid sequences of acetone-induced proteins. The acmA and acmB genes encode a Baeyer-Villiger monooxygenase (BVMO) and esterase, respectively. The BVMO encoded by acmA was purified from acetone-induced cells of Gordonia sp. strain TY-5 and characterized. The BVMO exhibited NADPH-dependent oxidation activity for linear ketones (C3 to C10) and cyclic ketones (C4 to C8). Escherichia coli expressing the acmA gene oxidized acetone to methyl acetate, and E. coli expressing the acmB gene hydrolyzed methyl acetate. Northern blot analyses revealed that polycistronic transcription of the acmAB gene cluster was induced by propane, 2-propanol, and acetone. These results indicate that the acmAB gene products play an important role in the metabolism of acetone derived from propane oxidation and clarify the propane metabolism pathway of strain TY-5 (propane --> 2-propanol --> acetone --> methyl acetate --> acetic acid + methanol). This paper provides the first evidence for BVMO-dependent acetone metabolism.
Figures


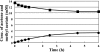


Comment in
-
New insights into acetone metabolism.J Bacteriol. 2007 Feb;189(3):671-3. doi: 10.1128/JB.01578-06. Epub 2006 Dec 1. J Bacteriol. 2007. PMID: 17142393 Free PMC article. No abstract available.
Similar articles
-
Kinetic characterization of acetone monooxygenase from Gordonia sp. strain TY-5.AMB Express. 2018 Nov 3;8(1):181. doi: 10.1186/s13568-018-0709-x. AMB Express. 2018. PMID: 30392152 Free PMC article.
-
Propane monooxygenase and NAD+-dependent secondary alcohol dehydrogenase in propane metabolism by Gordonia sp. strain TY-5.J Bacteriol. 2003 Dec;185(24):7120-8. doi: 10.1128/JB.185.24.7120-7128.2003. J Bacteriol. 2003. PMID: 14645271 Free PMC article.
-
New insights into acetone metabolism.J Bacteriol. 2007 Feb;189(3):671-3. doi: 10.1128/JB.01578-06. Epub 2006 Dec 1. J Bacteriol. 2007. PMID: 17142393 Free PMC article. No abstract available.
-
Gene structure and regulation of alkane monooxygenases in propane-utilizing Mycobacterium sp. TY-6 and Pseudonocardia sp. TY-7.J Biosci Bioeng. 2006 Sep;102(3):184-92. doi: 10.1263/jbb.102.184. J Biosci Bioeng. 2006. PMID: 17046531
-
Selective bio-oxidation of propane to acetone using methane-oxidizing Methylomonas sp. DH-1.J Ind Microbiol Biotechnol. 2017 Jul;44(7):1097-1105. doi: 10.1007/s10295-017-1936-x. Epub 2017 Mar 20. J Ind Microbiol Biotechnol. 2017. PMID: 28321646
Cited by
-
Microbial Biodegradation of Paraffin Wax in Malaysian Crude Oil Mediated by Degradative Enzymes.Front Microbiol. 2020 Sep 8;11:565608. doi: 10.3389/fmicb.2020.565608. eCollection 2020. Front Microbiol. 2020. PMID: 33013795 Free PMC article.
-
Kinetic characterization of acetone monooxygenase from Gordonia sp. strain TY-5.AMB Express. 2018 Nov 3;8(1):181. doi: 10.1186/s13568-018-0709-x. AMB Express. 2018. PMID: 30392152 Free PMC article.
-
Verrucomicrobial methanotrophs grow on diverse C3 compounds and use a homolog of particulate methane monooxygenase to oxidize acetone.ISME J. 2021 Dec;15(12):3636-3647. doi: 10.1038/s41396-021-01037-2. Epub 2021 Jun 22. ISME J. 2021. PMID: 34158629 Free PMC article.
-
Engineering Escherichia coli for Microbial Production of Butanone.Appl Environ Microbiol. 2016 Apr 18;82(9):2574-2584. doi: 10.1128/AEM.03964-15. Print 2016 May. Appl Environ Microbiol. 2016. PMID: 26896132 Free PMC article.
-
Whole Genome Analysis and Assessment of the Metabolic Potential of Gordonia rubripertincta Strain 112, a Degrader of Aromatic and Aliphatic Compounds.Biology (Basel). 2023 May 15;12(5):721. doi: 10.3390/biology12050721. Biology (Basel). 2023. PMID: 37237534 Free PMC article.
References
-
- Allen, J. E., F. W. Forney, and A. J. Markovetz. 1971. Microbial subterminal oxidation of alkanes and alk-1-enes. Lipids 6:448-452. - PubMed
-
- Ashraf, W., A. Mihdhir, and J. C. Murrell. 1994. Bacterial oxidation of propane. FEMS Microbiol. Lett. 122:1-6. - PubMed
-
- Birks, S. J., and D. L. Kelly. 1997. Assay and properties of acetone carboxylase, a novel enzyme involved in acetone-dependent growth and CO2 fixation in Rhodobacter capsulatus and other photosynthetic and denitrifying bacteria. Microbiology 143:755-766. - PubMed
-
- Bondoc, F. Y., Z. Bao, W.-Y. Hu, F. J. Gonzalez, Y. Wang, C. S. Yang, and J.-Y. Hong. 1999. Acetone catabolism by cytochrome P450 2E1: studies with CYP2E1-null mice. Biochem. Pharmacol. 58:461-463. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

