Electron-transfer reagent anion formation via electrospray ionization and collision-induced dissociation
- PMID: 17073403
- PMCID: PMC3168780
- DOI: 10.1021/ac061409v
Electron-transfer reagent anion formation via electrospray ionization and collision-induced dissociation
Abstract
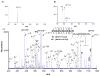
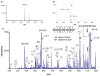
A strategy is described and demonstrated for the formation of reagent anions via electrospray ionization (ESI) for electron-transfer dissociation (ETD). To circumvent difficulties associated with formation of high mass-to-charge ratio (m/z) reagent anions, it is desirable to form ETD reagents via means other than those that require reagent molecule vaporization. ESI is a candidate method, but anions that are generally generated efficiently by ESI tend to react with multiply protonated polypeptides via proton transfer. The strategy described herein involves the use of a precursor reagent molecule that ionizes efficiently via electrospray ionization and that can subsequently be converted to an ETD reagent via gas-phase dissociation. The approach is demonstrated with arenecarboxylic acids that yield strong signals associated with the deprotonated molecule and that subsequently undergo collision-induced dissociation (CID) by loss of CO(2). In the present work, triply protonated KGAILKGAILR served as a test substrate for the CID product ions to give rise to ETD. Several precursor molecules were shown to be capable of generating ETD reagents via ESI followed by CID. These included 9-anthracenecarboxylic acid, 2-fluoro-5-iodobenzoic acid, and 2-(fluoranthene-8-carbonyl)benzoic acid. The latter molecule has the most attractive set of characteristics as a precursor for a relatively high m/z ratio ETD reagent.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

