Acute and chronic changes in aquaporin 4 expression after spinal cord injury
- PMID: 17074445
- PMCID: PMC1894918
- DOI: 10.1016/j.neuroscience.2006.08.079
Acute and chronic changes in aquaporin 4 expression after spinal cord injury
Abstract
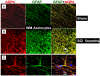
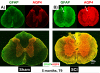
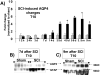
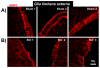
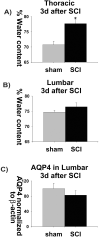
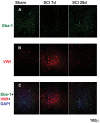
The effect of spinal cord injury (SCI) on the expression levels and distribution of water channel aquaporin 4 (AQP4) has not been studied. We have found AQP4 in gray and white matter astrocytes in both uninjured and injured rat spinal cords. AQP4 was detected in astrocytic processes that were tightly surrounding neurons and blood vessels, but more robustly in glia limitans externa and interna, which were forming an interface between spinal cord parenchyma and cerebrospinal fluid (CSF). Such spatial distribution of AQP4 suggests a critical role that astrocytes expressing AQP4 play in the transport of water from blood/CSF to spinal cord parenchyma and vice versa. SCI induced biphasic changes in astrocytic AQP4 levels, including its early down-regulation and subsequent persistent up-regulation. However, changes in AQP4 expression did not correlate well with the onset and magnitude of astrocytic activation, when measured as changes in GFAP expression levels. It appears that reactive astrocytes began expressing increased levels of AQP4 after migrating to the wound area (thoracic region) two weeks after SCI, and AQP4 remained significantly elevated for months after SCI. We also showed that increased levels of AQP4 spread away from the lesion site to cervical and lumbar segments, but only in chronically injured spinal cords. Although overall AQP4 expression levels increased in chronically-injured spinal cords, AQP4 immunolabeling in astrocytic processes forming glia limitans externa was decreased, which may indicate impaired water transport through glia limitans externa. Finally, we also showed that SCI-induced changes in AQP4 protein levels correlate, both temporally and spatially, with persistent increases in water content in acutely and chronically injured spinal cords. Although correlative, this finding suggests a possible link between AQP4 and impaired water transport/edema/syringomyelia in contused spinal cords.
Figures






Similar articles
-
Aquaporin 1 - a novel player in spinal cord injury.J Neurochem. 2008 May;105(3):628-40. doi: 10.1111/j.1471-4159.2007.05177.x. Epub 2008 Jan 28. J Neurochem. 2008. PMID: 18248364 Free PMC article.
-
Aquaporin-4 expression and blood-spinal cord barrier permeability in canalicular syringomyelia.J Neurosurg Spine. 2012 Dec;17(6):602-12. doi: 10.3171/2012.9.SPINE1265. Epub 2012 Oct 19. J Neurosurg Spine. 2012. PMID: 23082850
-
Aquaporins in spinal cord injury: the janus face of aquaporin 4.Neuroscience. 2010 Jul 28;168(4):1019-35. doi: 10.1016/j.neuroscience.2010.01.037. Epub 2010 Jan 28. Neuroscience. 2010. PMID: 20109536 Free PMC article. Review.
-
Inhibiting High Mobility Group Box-1 Reduces Early Spinal Cord Edema and Attenuates Astrocyte Activation and Aquaporin-4 Expression after Spinal Cord Injury in Rats.J Neurotrauma. 2019 Feb 1;36(3):421-435. doi: 10.1089/neu.2018.5642. Epub 2018 Oct 4. J Neurotrauma. 2019. PMID: 29929431
-
The Role of Aquaporins in Spinal Cord Injury.Cells. 2023 Jun 23;12(13):1701. doi: 10.3390/cells12131701. Cells. 2023. PMID: 37443735 Free PMC article. Review.
Cited by
-
Transporters and channels in cytotoxic astrocyte swelling.Neurochem Res. 2012 Nov;37(11):2379-87. doi: 10.1007/s11064-012-0777-2. Epub 2012 Apr 28. Neurochem Res. 2012. PMID: 22544664 Review.
-
Aquaporins in cerebrovascular disease: a target for treatment of brain edema?Cerebrovasc Dis. 2011;31(6):521-31. doi: 10.1159/000324328. Epub 2011 Apr 12. Cerebrovasc Dis. 2011. PMID: 21487216 Free PMC article. Review.
-
miR-320a affects spinal cord edema through negatively regulating aquaporin-1 of blood-spinal cord barrier during bimodal stage after ischemia reperfusion injury in rats.BMC Neurosci. 2016 Feb 5;17:10. doi: 10.1186/s12868-016-0243-1. BMC Neurosci. 2016. Retraction in: BMC Neurosci. 2021 Nov 3;22(1):64. doi: 10.1186/s12868-021-00669-6. PMID: 26850728 Free PMC article. Retracted.
-
Escalate and De-Escalate Therapies for Intracranial Pressure Control in Traumatic Brain Injury.Front Neurol. 2020 Nov 24;11:564751. doi: 10.3389/fneur.2020.564751. eCollection 2020. Front Neurol. 2020. PMID: 33324317 Free PMC article. Review.
-
Aquaporin 1 - a novel player in spinal cord injury.J Neurochem. 2008 May;105(3):628-40. doi: 10.1111/j.1471-4159.2007.05177.x. Epub 2008 Jan 28. J Neurochem. 2008. PMID: 18248364 Free PMC article.
References
-
- Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci. 2003;4:991–1001. - PubMed
-
- Amiry-Moghaddam M, Xue R, Haug FM, Neely JD, Bhardwaj A, Agre P, Adams ME, Froehner SC, Mori S, Ottersen OP. Alpha-syntrophin deletion removes the perivascular but not endothelial pool of aquaporin-4 at the blood-brain barrier and delays the development of brain edema in an experimental model of acute hyponatremia. FASEB J. 2004 Mar;18(3):542–4. - PubMed
-
- Badaut J, Lasbennes F, Magistretti PJ, Regli L. Aquaporins in brain: distribution, physiology, and pathophysiology. J Cereb Blood Flow Metab. 2002 Apr;22(4):367–78. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using NYU weight drop device versus transection. Exp. Neurol. 1996;139:244–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

