Electron detachment dissociation of glycosaminoglycan tetrasaccharides
- PMID: 17074503
- PMCID: PMC1784114
- DOI: 10.1016/j.jasms.2006.09.020
Electron detachment dissociation of glycosaminoglycan tetrasaccharides
Abstract
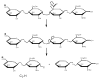
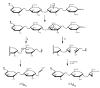
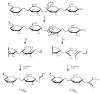
The first application of electron detachment dissociation (EDD) to carbohydrates is presented. The structural characterization of glycosaminoglycan (GAG) oligosaccharides by mass spectrometry is a longstanding problem because of the lability of these acidic, polysulfated carbohydrates. Doubly-charged negative ions of four GAG tetrasaccharides are examined by EDD, collisionally activated dissociation (CAD), and infrared multiphoton dissociation (IRMPD). EDD is found to produce information-rich mass spectra with both cross ring and glycosidic cleavage product ions. In contrast, most of the product ions produced by CAD and IRMPD result from glycosidic cleavage. EDD shows great potential as a tool for locating the sites of sulfation and other modifications in glycosaminoglycan oligosaccharides.
Figures





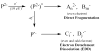
Similar articles
-
Electron-induced dissociation of glycosaminoglycan tetrasaccharides.J Am Soc Mass Spectrom. 2008 Oct;19(10):1449-58. doi: 10.1016/j.jasms.2008.06.024. Epub 2008 Jul 2. J Am Soc Mass Spectrom. 2008. PMID: 18657442 Free PMC article.
-
Electron capture dissociation, electron detachment dissociation and infrared multiphoton dissociation of sucrose octasulfate.Eur J Mass Spectrom (Chichester). 2009;15(2):275-81. doi: 10.1255/ejms.951. Eur J Mass Spectrom (Chichester). 2009. PMID: 19423912 Free PMC article.
-
Distinguishing glucuronic from iduronic acid in glycosaminoglycan tetrasaccharides by using electron detachment dissociation.Anal Chem. 2007 Mar 1;79(5):2015-22. doi: 10.1021/ac061636x. Epub 2007 Jan 25. Anal Chem. 2007. PMID: 17253657 Free PMC article.
-
Electron photodetachment dissociation for structural characterization of synthetic and bio-polymer anions.Mass Spectrom Rev. 2014 Nov-Dec;33(6):501-22. doi: 10.1002/mas.21402. Epub 2013 Nov 27. Mass Spectrom Rev. 2014. PMID: 24285407 Review.
-
Gas-Phase Fragmentation of Cyclic Oligosaccharides in Tandem Mass Spectrometry.Molecules. 2019 Jun 14;24(12):2226. doi: 10.3390/molecules24122226. Molecules. 2019. PMID: 31207901 Free PMC article. Review.
Cited by
-
Electron detachment dissociation of underivatized chloride-adducted oligosaccharides.J Am Soc Mass Spectrom. 2012 Nov;23(11):2031-42. doi: 10.1007/s13361-012-0459-y. Epub 2012 Aug 22. J Am Soc Mass Spectrom. 2012. PMID: 22911097
-
LC-MS and LC-MS/MS studies of incorporation of 34SO3 into glycosaminoglycan chains by sulfotransferases.Glycobiology. 2013 Aug;23(8):969-79. doi: 10.1093/glycob/cwt033. Epub 2013 May 21. Glycobiology. 2013. PMID: 23696150 Free PMC article.
-
Heparin and heparan sulfate: analyzing structure and microheterogeneity.Handb Exp Pharmacol. 2012;(207):159-76. doi: 10.1007/978-3-642-23056-1_8. Handb Exp Pharmacol. 2012. PMID: 22566225 Free PMC article. Review.
-
On-line separations combined with MS for analysis of glycosaminoglycans.Mass Spectrom Rev. 2009 Mar-Apr;28(2):254-72. doi: 10.1002/mas.20200. Mass Spectrom Rev. 2009. PMID: 18956477 Free PMC article. Review.
-
Proteoglycan sequence.Mol Biosyst. 2012 Jun;8(6):1613-25. doi: 10.1039/c2mb25021g. Epub 2012 Apr 19. Mol Biosyst. 2012. PMID: 22513887 Free PMC article. Review.
References
-
- Perrimon N, Bernfield M. Cellular functions of proteoglycans--an overview. Semin Cell Dev Biol. 2001;12:65–67. - PubMed
-
- Linhardt RJ, Toida T. Role of Glycosaminoglycans in Cellular Communication. Acc Chem Res. 2004;37:431–438. - PubMed
-
- Fannon M, Forsten KE, Nugent MA. Potentiation and Inhibition of bFGF Binding by Heparin: A Model for Regulation of Cellular Response. Biochemistry. 2000;39:1434–1445. - PubMed
-
- Wu ZL, Zhang L, Yabe T, Kuberan B, Beeler DL, Love A, Rosenberg RD. The Involvement of Heparan Sulfate (HS) in FGF1/HS/FGFR1 Signaling Complex. J Biol Chem. 2003;278:17121–17129. - PubMed
-
- Gotte M. Syndecans in Inflammation. FASEB J. 2003;17:575–591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

