Structure and biosynthesis of heat-stable antifungal factor (HSAF), a broad-spectrum antimycotic with a novel mode of action
- PMID: 17074795
- PMCID: PMC1797680
- DOI: 10.1128/AAC.00931-06
Structure and biosynthesis of heat-stable antifungal factor (HSAF), a broad-spectrum antimycotic with a novel mode of action
Abstract
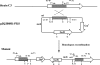
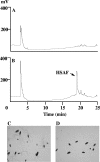
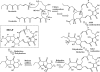
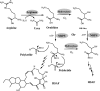
A screen for antifungal compounds from Lysobacter enzymogenes strain C3, a bacterial biological control agent of fungal diseases, has previously led to the isolation of heat-stable antifungal factor (HSAF). HSAF exhibits inhibitory activities against a wide range of fungal species and shows a novel mode of antifungal action by disrupting the biosynthesis of a distinct group of sphingolipids. We have now determined the chemical structure of HSAF, which is identical to that of dihydromaltophilin, an antifungal metabolite with a unique macrocyclic lactam system containing a tetramic acid moiety and a 5,5,6-tricyclic skeleton. We have also identified the genetic locus responsible for the biosynthesis of HSAF in strain C3. DNA sequencing of this locus revealed genes for a hybrid polyketide synthase-nonribosomal peptide synthetase (PKS-NRPS), a sterol desaturase, a ferredoxin reductase, and an arginase. The disruption of the PKS-NRPS gene generated C3 mutants that lost the ability to produce HSAF and to inhibit fungal growth, demonstrating a hybrid PKS-NRPS that catalyzed the biosynthesis of the unique macrolactam system that is found in many biologically active natural products isolated from marine organisms. In addition, we have generated mutants with disrupted sterol desaturase, ferredoxin reductase, and arginase and examined the metabolites produced in these mutants. The work represents the first study of the genetic basis for the biosynthesis of the tetramic acid-containing macrolactams. The elucidation of the chemical structure of HSAF and the identification of the genetic locus for its biosynthesis establish the foundation for future exploitation of this group of compounds as new fungicides or antifungal drugs.
Figures




References
-
- Ash, D. E. 2004. Structure and function of arginases. J. Nutr. 134:2760S-2764S. - PubMed
-
- Boeckman, R. K. J., C. H. Weidner, R. B. Perni, and J. J. Napier. 1989. An enantioselective and highly convergent synthesis of (+)-ikarugamycin. J. Am. Chem. Soc. 111:8036-8037.
-
- Cramer, N., M. Buchweitz, S. Laschat, W. Frey, A. Baro, D. Mathieu, C. Richter, and H. Schwalbe. 2006. Total synthesis and NMR investigations of cylindramide. Chemistry 12:2488-2503. - PubMed
-
- Cramer, N., S. Laschat, A. Baro, H. Schwalbe, and C. Richter. 2005. Enantioselective total synthesis of cylindramide. Angew. Chem. Int. Ed. Engl. 44:820-822. - PubMed
-
- Du, L., Y. Cheng, G. Ingenhorst, G. Tang, Y. Huang, and B. Shen. 2003. Hybrid peptide-polyketide natural products: biosynthesis and prospects towards engineering novel molecules, p. 227-267. In J. K. Setlow (ed.), Genetic engineering—principles and methods, vol. 25. Kluwer Academic/Plenum Publishers, New York, NY. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

