Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells
- PMID: 17074804
- PMCID: PMC1800800
- DOI: 10.1128/MCB.01539-06
Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells
Abstract
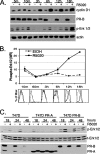
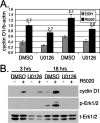
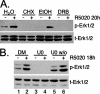
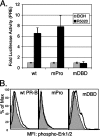
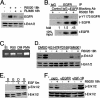
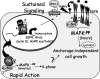
Progesterone receptor (PR) ligand binding induces rapid and transient (5- to 10-min) activation of cytosolic c-Src-Ras-Erk1/2 mitogen-activated protein kinase (MAPK) signaling that is independent of PR functioning as transcription factors. Here, we have explored the integration of PR-dependent transcription and rapid signaling events in breast cancer cells. PR-B, but not PR-A, induced robust and sustained (6- to 72-h) Erk1/2 activation that was required for elevated cyclin D1 protein but not mRNA levels. Sustained Erk1/2 activation in response to progestins occurred via a novel mechanism distinct from rapid signaling initiated by PR/c-Src interactions and required the PR-B DNA-binding domain (DBD). PR/progestin upregulated epidermal growth factor receptor (EGFR) and Wnt-1. In response to PR-induced Wnt-1 signaling, matrix metalloprotease (MMP)-mediated membrane-proximal shedding of EGFR ligands transactivated EGFR and induced persistent downstream c-Src and Erk1/2 activities. T47D cell anchorage-independent growth was stimulated by progestins and blocked by inhibition of Erk1/2, c-Src, EGFR, or RNA interference of Wnt-1. Similarly, cell growth in soft agar required the PR DBD but was sensitive to disruption of PR/c-Src interactions, suggesting that both PR-B-induced rapid signaling events and nuclear actions contribute to this response. Our discovery that progestins are capable of robust autocrine activation of EGFR and sustained Erk1/2 signaling provides further support for the physiological linkage of growth factor and steroid hormone signaling. PR-B-induced sustained MAPK signaling may provide prosurvival or proliferative advantages to early breast cancer lesions.
Figures



References
-
- Aupperlee, M. D., K. T. Smith, A. Kariagina, and S. Z. Haslam. 2005. Progesterone receptor isoforms A and B: temporal and spatial differences in expression during murine mammary gland development. Endocrinology 146:3577-3588. - PubMed
-
- Beral, V. 2003. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 362:419-427. - PubMed
-
- Bertics, P. J., W. S. Chen, L. Hubler, C. S. Lazar, M. G. Rosenfeld, and G. N. Gill. 1988. Alteration of epidermal growth factor receptor activity by mutation of its primary carboxyl-terminal site of tyrosine self-phosphorylation. J. Biol. Chem. 263:3610-3617. - PubMed
-
- Biscardi, J. S., M. C. Maa, D. A. Tice, M. E. Cox, T. H. Leu, and S. J. Parsons. 1999. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J. Biol. Chem. 274:8335-8343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
