BiP internal ribosomal entry site activity is controlled by heat-induced interaction of NSAP1
- PMID: 17074807
- PMCID: PMC1800651
- DOI: 10.1128/MCB.00814-06
BiP internal ribosomal entry site activity is controlled by heat-induced interaction of NSAP1
Abstract
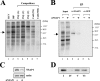
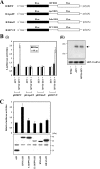
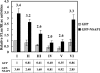
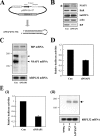
TheBiP protein, a stress response protein, plays an important role in the proper folding and assembly of nascent protein and in the scavenging of misfolded proteins in the endoplasmic reticulum lumen. Translation of BiP is directed by an internal ribosomal entry site (IRES) in the 5' nontranslated region of the BiP mRNA. BiP IRES activity increases when cells are heat stressed. Here we report that NSAP1 specifically enhances the IRES activity of BiP mRNA by interacting with the IRES element. Overexpression of NSAP1 in 293T cells increased the IRES activity of BiP mRNA, whereas knockdown of NSAP1 by small interfering RNA (siRNA) reduced the IRES activity of BiP mRNA. The amount of NSAP1 bound to the BiP IRES increased under heat stress conditions, and the IRES activity of BiP mRNA was increased. Moreover, the increase in BiP IRES activity with heat treatment was not observed in cells lacking NSAP1 after siRNA treatment. BiP mRNAs were redistributed from the heavy polysome to the light polysome in NSAP1 knockdown cells. Together, these data indicate that NSAP1 modulates IRES-dependent translation of BiP mRNA through an RNA-protein interaction under heat stress conditions.
Figures



Similar articles
-
Polypyrimidine tract-binding protein inhibits translation of bip mRNA.J Mol Biol. 2000 Nov 24;304(2):119-33. doi: 10.1006/jmbi.2000.4179. J Mol Biol. 2000. PMID: 11080450
-
RNA-binding protein hnRNP D modulates internal ribosome entry site-dependent translation of hepatitis C virus RNA.J Virol. 2008 Dec;82(24):12082-93. doi: 10.1128/JVI.01405-08. Epub 2008 Oct 8. J Virol. 2008. PMID: 18842733 Free PMC article.
-
Continuous heat shock enhances translational initiation directed by internal ribosomal entry site.Biochem Biophys Res Commun. 2002 Sep 20;297(2):224-31. doi: 10.1016/s0006-291x(02)02154-x. Biochem Biophys Res Commun. 2002. PMID: 12237106
-
Heat-shock proteins as regulators of apoptosis.Oncogene. 2003 Dec 8;22(56):9041-7. doi: 10.1038/sj.onc.1207114. Oncogene. 2003. PMID: 14663482 Review.
-
Functions and pathologies of BiP and its interaction partners.Cell Mol Life Sci. 2009 May;66(9):1556-69. doi: 10.1007/s00018-009-8745-y. Cell Mol Life Sci. 2009. PMID: 19151922 Free PMC article. Review.
Cited by
-
Human cytomegalovirus induces the endoplasmic reticulum chaperone BiP through increased transcription and activation of translation by using the BiP internal ribosome entry site.J Virol. 2010 Nov;84(21):11479-86. doi: 10.1128/JVI.01330-10. Epub 2010 Aug 25. J Virol. 2010. PMID: 20739513 Free PMC article.
-
WHEP domains direct noncanonical function of glutamyl-Prolyl tRNA synthetase in translational control of gene expression.Mol Cell. 2008 Mar 28;29(6):679-90. doi: 10.1016/j.molcel.2008.01.010. Mol Cell. 2008. PMID: 18374644 Free PMC article.
-
Induction of endoplasmic reticulum stress genes, BiP and chop, in genetic and environmental models of retinal degeneration.Invest Ophthalmol Vis Sci. 2012 Nov 9;53(12):7590-9. doi: 10.1167/iovs.12-10221. Invest Ophthalmol Vis Sci. 2012. PMID: 23074209 Free PMC article.
-
Translational upregulation of Aurora-A by hnRNP Q1 contributes to cell proliferation and tumorigenesis in colorectal cancer.Cell Death Dis. 2017 Jan 12;8(1):e2555. doi: 10.1038/cddis.2016.479. Cell Death Dis. 2017. PMID: 28079881 Free PMC article.
-
GRP78 at the Centre of the Stage in Cancer and Neuroprotection.Front Neurosci. 2017 Apr 5;11:177. doi: 10.3389/fnins.2017.00177. eCollection 2017. Front Neurosci. 2017. PMID: 28424579 Free PMC article. Review.
References
-
- Bannai, H., K. Fukatsu, A. Mizutani, T. Natsume, S. I. Iemura, T. Ikegami, T. Inoue, and K. Mikoshiba. 2004. An RNA-interacting protein, SYNCRIP (hnRNP Q1/NSAP1) is a component of mRNA granule transported with inositol 1,4,5-trisphosphate receptor type 1 mRNA in neuronal dendrites. J. Biol. Chem. 279:53427-53434. - PubMed
-
- Duncan, R. F. 1996. Translational control during heat shock, p. 271-293. In J. W. B. Hershey, M. B. Mathews, and N. Sonenberg (ed.), Translational control. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
-
- Gabai, V. L., and M. Y. Sherman. 2002. Invited review: interplay between molecular chaperones and signaling pathways in survival of heat shock. J. Appl. Physiol. 92:1743-1748. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
