Targeted deletion of the muscular dystrophy gene myotilin does not perturb muscle structure or function in mice
- PMID: 17074808
- PMCID: PMC1800670
- DOI: 10.1128/MCB.00561-06
Targeted deletion of the muscular dystrophy gene myotilin does not perturb muscle structure or function in mice
Abstract
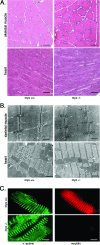
Myotilin, palladin, and myopalladin form a novel small subfamily of cytoskeletal proteins that contain immunoglobulin-like domains. Myotilin is a thin filament-associated protein localized at the Z-disk of skeletal and cardiac muscle cells. The direct binding to F-actin, efficient cross-linking of actin filaments, and prevention of induced disassembly of filaments are key roles of myotilin that are thought to be involved in structural maintenance and function of the sarcomere. Missense mutations in the myotilin-encoding gene cause dominant limb girdle muscular dystrophy type 1A and spheroid body myopathy and are the molecular defect that can cause myofibrillar myopathy. Here we describe the generation and analysis of mice that lack myotilin, myo(-/-) mice. Surprisingly, myo(-/-) mice maintain normal muscle sarcomeric and sarcolemmal integrity. Also, loss of myotilin does not cause alterations in the heart or other organs of newborn or adult myo(-/-) mice. The mice develop normally and have a normal life span, and their muscle capacity does not significantly differ from wild-type mice even after prolonged physical stress. The results suggest that either myotilin does not participate in muscle development and basal function maintenance or other proteins serve as structural and functional compensatory molecules when myotilin is absent.
Figures

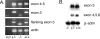


Similar articles
-
Myotilin, a novel sarcomeric protein with two Ig-like domains, is encoded by a candidate gene for limb-girdle muscular dystrophy.Hum Mol Genet. 1999 Jul;8(7):1329-36. doi: 10.1093/hmg/8.7.1329. Hum Mol Genet. 1999. PMID: 10369880
-
Maintenance of muscle mass, fiber size, and contractile function in mice lacking the Z-disc protein myotilin.Ups J Med Sci. 2009;114(4):235-41. doi: 10.3109/03009730903276399. Ups J Med Sci. 2009. PMID: 19878039 Free PMC article.
-
Z-disc-associated, alternatively spliced, PDZ motif-containing protein (ZASP) mutations in the actin-binding domain cause disruption of skeletal muscle actin filaments in myofibrillar myopathy.J Biol Chem. 2014 May 9;289(19):13615-26. doi: 10.1074/jbc.M114.550418. Epub 2014 Mar 25. J Biol Chem. 2014. PMID: 24668811 Free PMC article.
-
The palladin/myotilin/myopalladin family of actin-associated scaffolds.Int Rev Cytol. 2005;246:31-58. doi: 10.1016/S0074-7696(05)46002-7. Int Rev Cytol. 2005. PMID: 16164966 Review.
-
Tropomodulin capping of actin filaments in striated muscle development and physiology.J Biomed Biotechnol. 2011;2011:103069. doi: 10.1155/2011/103069. Epub 2011 Oct 17. J Biomed Biotechnol. 2011. PMID: 22013379 Free PMC article. Review.
Cited by
-
RNAi-mediated Gene Silencing of Mutant Myotilin Improves Myopathy in LGMD1A Mice.Mol Ther Nucleic Acids. 2014 Apr 29;3(4):e160. doi: 10.1038/mtna.2014.13. Mol Ther Nucleic Acids. 2014. PMID: 24781192 Free PMC article.
-
Molecular basis of F-actin regulation and sarcomere assembly via myotilin.PLoS Biol. 2021 Apr 12;19(4):e3001148. doi: 10.1371/journal.pbio.3001148. eCollection 2021 Apr. PLoS Biol. 2021. PMID: 33844684 Free PMC article.
-
Dominantly inherited muscle disorders: understanding their complexity and exploring therapeutic approaches.Dis Model Mech. 2024 Oct 1;17(10):dmm050720. doi: 10.1242/dmm.050720. Epub 2024 Nov 6. Dis Model Mech. 2024. PMID: 39501809 Free PMC article. Review.
-
Conformational plasticity and evolutionary analysis of the myotilin tandem Ig domains.Sci Rep. 2017 Jun 21;7(1):3993. doi: 10.1038/s41598-017-03323-6. Sci Rep. 2017. PMID: 28638118 Free PMC article.
-
Loss of enigma homolog protein results in dilated cardiomyopathy.Circ Res. 2010 Aug 6;107(3):348-56. doi: 10.1161/CIRCRESAHA.110.218735. Epub 2010 Jun 10. Circ Res. 2010. PMID: 20538684 Free PMC article.
References
-
- Agbulut, O., J. Destombes, D. Thiesson, and G. Butler-Browne. 2000. Age-related appearance of tubular aggregates in the skeletal muscle of almost all male inbred mice. Histochem. Cell Biol. 114:477-481. - PubMed
-
- Allen, D. L., B. C. Harrison, A. Maass, M. L. Bell, W. C. Byrnes, and L. A. Leinwand. 2001. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J. Appl. Physiol. 90:1900-1908. - PubMed
-
- Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. - PubMed
-
- Connolly, A. M., R. M. Keeling, S. Mehta, A. Pestronk, and J. R. Sanes. 2001. Three mouse models of muscular dystrophy: the natural history of strength and fatigue in dystrophin-, dystrophin/utrophin-, and laminin α2-deficient mice. Neuromuscul. Disord. 11:703-712. - PubMed
-
- Faulkner, G., A. Pallavicini, A. Comelli, M. Salamon, G. Bortoletto, C. Ievolella, S. Trevisan, S. Kojic, F. Dalla Vecchia, P. Laveder, G. Valle, and G. Lanfranchi. 2000. FATZ, a filamin-, actinin-, and telethonin-binding protein of the Z-disc of skeletal muscle. J. Biol. Chem. 275:41234-41242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
