Dynamic interplay of transcriptional machinery and chromatin regulates "late" expression of the chemokine RANTES in T lymphocytes
- PMID: 17074812
- PMCID: PMC1800668
- DOI: 10.1128/MCB.01071-06
Dynamic interplay of transcriptional machinery and chromatin regulates "late" expression of the chemokine RANTES in T lymphocytes
Abstract
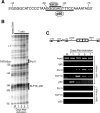
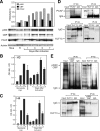
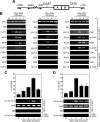
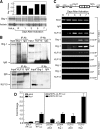
The chemokine RANTES (regulated upon activation normal T cell expressed and secreted) is expressed "late" (3 to 5 days) after activation in T lymphocytes. In order to understand the molecular events that accompany changes in gene expression, a detailed analysis of the interplay between transcriptional machinery and chromatin on the RANTES promoter over time was undertaken. Krüppel-like factor 13 (KLF13), a sequence-specific DNA binding transcription factor, orchestrates the induction of RANTES expression in T lymphocytes by ordered recruitment of effector molecules, including Nemo-like kinase, p300/cyclic AMP response element binding protein (CBP), p300/CBP-associated factor, and Brahma-related gene 1, that initiate sequential changes in phosphorylation and acetylation of histones and ATP-dependent chromatin remodeling near the TATA box of the RANTES promoter. These events recruit RNA polymerase II to the RANTES promoter and are responsible for late expression of RANTES in T lymphocytes. Therefore, KLF13 is a key regulator of late RANTES expression in T lymphocytes.
Figures


References
-
- Agalioti, T., G. Chen, and D. Thanos. 2002. Deciphering the transcriptional histone acetylation code for a human gene. Cell 111:381-392. - PubMed
-
- Asano, H., X. S. Li, and G. Stamatoyannopoulos. 2000. FKLF-2: a novel Kruppel-like transcriptional factor that activates globin and other erythroid lineage genes. Blood 95:3578-3584. - PubMed
-
- Berger, S. L. 2002. Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev. 12:142-148. - PubMed
-
- Beverly, L. J., and A. J. Capobianco. 2004. Targeting promiscuous signaling pathways in cancer: another Notch in the bedpost. Trends Mol. Med. 10:591-598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
