Mechanisms of two modulatory actions of the channel-binding protein Slob on the Drosophila Slowpoke calcium-dependent potassium channel
- PMID: 17074977
- PMCID: PMC2151581
- DOI: 10.1085/jgp.200609653
Mechanisms of two modulatory actions of the channel-binding protein Slob on the Drosophila Slowpoke calcium-dependent potassium channel
Abstract
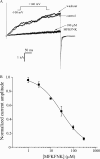
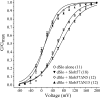
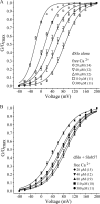
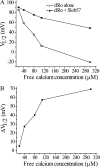
Slob57 is an ion channel auxiliary protein that binds to and modulates the Drosophila Slowpoke calcium-dependent potassium channel (dSlo). We reported recently that residues 1-39 of Slob57 comprise the key domain that both causes dSlo inactivation and shifts its voltage dependence of activation to more depolarized voltages. In the present study we show that removal of residues 2-6 from Slob57 abolishes the inactivation, but the ability of Slob57 to rightward shift the voltage dependence of activation of dSlo remains. A synthetic peptide corresponding in sequence to residues 1-6 of Slob57 blocks dSlo in a voltage- and dose-dependent manner. Two Phe residues and at least one Lys residue in this peptide are required for the blocking action. These data indicate that the amino terminus of Slob57 directly blocks dSlo, thereby leading to channel inactivation. Further truncation to residue Arg(16) eliminates the modulation of voltage dependence of activation. Thus these two modulatory actions of Slob57 are independent. Mutation within the calcium bowl of dSlo greatly reduces its calcium sensitivity (Bian, S., I. Favre, and E. Moczydlowski. 2001. Proc. Natl. Acad. Sci. USA. 98:4776-4781). We found that Slob57 still causes inactivation of this mutant channel, but does not shift its voltage dependence of activation. This result confirms further the independence of the inactivation and the voltage shift produced by Slob57. It also suggests that the voltage shift requires high affinity Ca(2+) binding to an intact calcium bowl. Furthermore, Slob57 inhibits the shift in the voltage dependence of activation of dSlo evoked by Ca(2+), and this inhibition by Slob57 is greater at higher free Ca(2+) concentrations. These results implicate distinct calcium-dependent and -independent mechanisms in the modulation of dSlo by Slob.
Figures






References
-
- Adelman, J.P., K.-Z. Shen, M.P. Kavanaugh, R.A. Warren, Y.-N. Wu, A. Lagrutta, C.T. Bond, and R.A. North. 1992. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 9:209–216. - PubMed
-
- Atkinson, N.S., G.A. Robertson, and B. Ganetzky. 1991. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 253:551–555. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

