Vitamin C mediates chemical aging of lens crystallins by the Maillard reaction in a humanized mouse model
- PMID: 17075057
- PMCID: PMC1636553
- DOI: 10.1073/pnas.0605101103
Vitamin C mediates chemical aging of lens crystallins by the Maillard reaction in a humanized mouse model
Abstract
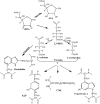
Senile cataracts are associated with progressive oxidation, fragmentation, cross-linking, insolubilization, and yellow pigmentation of lens crystallins. We hypothesized that the Maillard reaction, which leads browning and aroma development during the baking of foods, would occur between the lens proteins and the highly reactive oxidation products of vitamin C. To test this hypothesis, we engineered a mouse that selectively overexpresses the human vitamin C transporter SVCT2 in the lens. Consequently, lenticular levels of vitamin C and its oxidation products were 5- to 15-fold elevated, resulting in a highly compressed aging process and accelerated formation of several protein-bound advanced Maillard reaction products identical with those of aging human lens proteins. These data strongly implicate vitamin C in lens crystallin aging and may serve as a model for protein aging in other tissues particularly rich in vitamin C, such as the hippocampal neurons and the adrenal gland. The hSVCT2 mouse is expected to facilitate the search for drugs that inhibit damage by vitamin C oxidation products.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Topical application of L-arginine blocks advanced glycation by ascorbic acid in the lens of hSVCT2 transgenic mice.Mol Vis. 2011;17:2221-7. Epub 2011 Aug 18. Mol Vis. 2011. PMID: 21897744 Free PMC article.
-
Vitamin C-mediated Maillard reaction in the lens probed in a transgenic-mouse model.Ann N Y Acad Sci. 2008 Apr;1126:194-200. doi: 10.1196/annals.1433.064. Ann N Y Acad Sci. 2008. PMID: 18448816 Free PMC article.
-
Inhibition of crystallin ascorbylation by nucleophilic compounds in the hSVCT2 mouse model of lenticular aging.Invest Ophthalmol Vis Sci. 2008 Nov;49(11):4945-52. doi: 10.1167/iovs.08-1813. Epub 2008 Apr 17. Invest Ophthalmol Vis Sci. 2008. PMID: 18421088 Free PMC article.
-
Protein posttranslational modification (PTM) by glycation: Role in lens aging and age-related cataractogenesis.Exp Eye Res. 2021 Sep;210:108705. doi: 10.1016/j.exer.2021.108705. Epub 2021 Jul 20. Exp Eye Res. 2021. PMID: 34297945 Free PMC article. Review.
-
Protein oxidation and loss of protease activity may lead to cataract formation in the aged lens.Free Radic Biol Med. 1987;3(6):371-7. doi: 10.1016/0891-5849(87)90015-3. Free Radic Biol Med. 1987. PMID: 3322949 Review.
Cited by
-
Topical application of L-arginine blocks advanced glycation by ascorbic acid in the lens of hSVCT2 transgenic mice.Mol Vis. 2011;17:2221-7. Epub 2011 Aug 18. Mol Vis. 2011. PMID: 21897744 Free PMC article.
-
Vitamin C-mediated Maillard reaction in the lens probed in a transgenic-mouse model.Ann N Y Acad Sci. 2008 Apr;1126:194-200. doi: 10.1196/annals.1433.064. Ann N Y Acad Sci. 2008. PMID: 18448816 Free PMC article.
-
Oxidation as an important factor of protein damage: Implications for Maillard reaction.J Biosci. 2015 Jun;40(2):419-39. doi: 10.1007/s12038-015-9523-7. J Biosci. 2015. PMID: 25963268 Review.
-
Applied Healthspan engineering.Rejuvenation Res. 2010 Apr-Jun;13(2-3):265-80. doi: 10.1089/rej.2009.0969. Rejuvenation Res. 2010. PMID: 20462384 Free PMC article. Review.
-
Activation of unfolded protein response in transgenic mouse lenses.Invest Ophthalmol Vis Sci. 2011 Apr 4;52(5):2100-8. doi: 10.1167/iovs.10-5650. Print 2011 Apr. Invest Ophthalmol Vis Sci. 2011. PMID: 21310900 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

