Human immunodeficiency virus impairs reverse cholesterol transport from macrophages
- PMID: 17076584
- PMCID: PMC1629034
- DOI: 10.1371/journal.pbio.0040365
Human immunodeficiency virus impairs reverse cholesterol transport from macrophages
Abstract
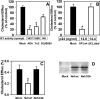
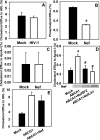
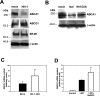
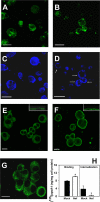
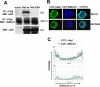
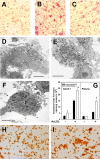
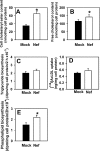
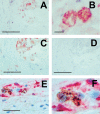
Several steps of HIV-1 replication critically depend on cholesterol. HIV infection is associated with profound changes in lipid and lipoprotein metabolism and an increased risk of coronary artery disease. Whereas numerous studies have investigated the role of anti-HIV drugs in lipodystrophy and dyslipidemia, the effects of HIV infection on cellular cholesterol metabolism remain uncharacterized. Here, we demonstrate that HIV-1 impairs ATP-binding cassette transporter A1 (ABCA1)-dependent cholesterol efflux from human macrophages, a condition previously shown to be highly atherogenic. In HIV-1-infected cells, this effect was mediated by Nef. Transfection of murine macrophages with Nef impaired cholesterol efflux from these cells. At least two mechanisms were found to be responsible for this phenomenon: first, HIV infection and transfection with Nef induced post-transcriptional down-regulation of ABCA1; and second, Nef caused redistribution of ABCA1 to the plasma membrane and inhibited internalization of apolipoprotein A-I. Binding of Nef to ABCA1 was required for down-regulation and redistribution of ABCA1. HIV-infected and Nef-transfected macrophages accumulated substantial amounts of lipids, thus resembling foam cells. The contribution of HIV-infected macrophages to the pathogenesis of atherosclerosis was supported by the presence of HIV-positive foam cells in atherosclerotic plaques of HIV-infected patients. Stimulation of cholesterol efflux from macrophages significantly reduced infectivity of the virions produced by these cells, and this effect correlated with a decreased amount of virion-associated cholesterol, suggesting that impairment of cholesterol efflux is essential to ensure proper cholesterol content in nascent HIV particles. These results reveal a previously unrecognized dysregulation of intracellular lipid metabolism in HIV-infected macrophages and identify Nef and ABCA1 as the key players responsible for this effect. Our findings have implications for pathogenesis of both HIV disease and atherosclerosis, because they reveal the role of cholesterol efflux impairment in HIV infectivity and suggest a possible mechanism by which HIV infection of macrophages may contribute to increased risk of atherosclerosis in HIV-infected patients.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures

Comment in
-
HIV-cholesterol connection suggests a new antiretroviral strategy.PLoS Biol. 2006 Nov;4(11):e400. doi: 10.1371/journal.pbio.0040400. Epub 2006 Oct 31. PLoS Biol. 2006. PMID: 20076496 Free PMC article. No abstract available.
References
-
- Maziere JC, Landureau JC, Giral P, Auclair M, Fall L, et al. Lovastatin inhibits HIV-1 expression in H9 human T lymphocytes cultured in cholesterol-poor medium. Biomed Pharmacother. 1994;48:63–67. - PubMed
-
- Campbell SM, Crowe SM, Mak J. Virion-associated cholesterol is critical for the maintenance of HIV-1 structure and infectivity. AIDS. 2002;16:2253–2261. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

