Quantum dot/peptide-MHC biosensors reveal strong CD8-dependent cooperation between self and viral antigens that augment the T cell response
- PMID: 17077145
- PMCID: PMC1636542
- DOI: 10.1073/pnas.0607771103
Quantum dot/peptide-MHC biosensors reveal strong CD8-dependent cooperation between self and viral antigens that augment the T cell response
Abstract
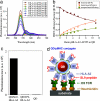
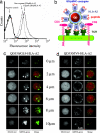
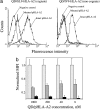
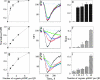
Cytotoxic T lymphocytes (CTL) can respond to a few viral peptide-MHC-I (pMHC-I) complexes among a myriad of virus-unrelated endogenous self pMHC-I complexes displayed on virus-infected cells. To elucidate the molecular recognition events on live CTL, we have utilized a self-assembled biosensor composed of semiconductor nanocrystals, quantum dots, carrying a controlled number of virus-derived (cognate) and other (noncognate) pMHC-I complexes and examined their recognition by antigen-specific T cell receptor (TCR) on anti-virus CD8(+) T cells. The unique architecture of nanoscale quantum dot/pMHC-I conjugates revealed that unexpectedly strong multivalent CD8-MHC-I interactions underlie the cooperative contribution of noncognate pMHC-I to the recognition of cognate pMHC-I by TCR to augment T cell responses. The cooperative, CD8-dependent spread of signal from a few productively engaged TCR to many other TCR can explain the remarkable ability of CTL to respond to virus-infected cells that present few cognate pMHC-I complexes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Rudolph MG, Wilson IA. Curr Opin Immunol. 2002;14:52–65. - PubMed
-
- Gao GF, Rao Z, Bell JI. Trends Immunol. 2002;23:408–413. - PubMed
-
- Hugues S, Malherbe L, Filippi C, Glaichenhaus N. J Immunol Methods. 2002;268:83–92. - PubMed
-
- Gestwicki JE, Cairo CW, Strong LE, Oetjen KA, Kiessling LL. J Am Chem Soc. 2002;124:14922–14933. - PubMed
-
- Mattoussi H, Mauro JM, Goldman ER, Anderson GP, Sundar VC, Mikulec FV, Bawendi MG. J Am Chem Soc. 2000;122:12142–12150.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

