Interactions between IL-32 and tumor necrosis factor alpha contribute to the exacerbation of immune-inflammatory diseases
- PMID: 17078892
- PMCID: PMC1794509
- DOI: 10.1186/ar2074
Interactions between IL-32 and tumor necrosis factor alpha contribute to the exacerbation of immune-inflammatory diseases
Abstract
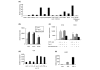
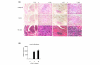
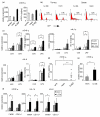
IL-32 is a newly described cytokine in the human found to be an in vitro inducer of tumor necrosis factor alpha (TNFalpha). We examined the in vivo relationship between IL-32 and TNFalpha, and the pathologic role of IL-32 in the TNFalpha-related diseases - arthritis and colitis. We demonstrated by quantitative PCR assay that IL-32 mRNA was expressed in the lymphoid tissues, and in stimulated peripheral T cells, monocytes, and B cells. Activated T cells were important for IL-32 mRNA expression in monocytes and B cells. Interestingly, TNFalpha reciprocally induced IL-32 mRNA expression in T cells, monocyte-derived dendritic cells, and synovial fibroblasts. Moreover, IL-32 mRNA expression was prominent in the synovial tissues of rheumatoid arthritis patients, especially in synovial-infiltrated lymphocytes by in situ hybridization. To examine the in vivo relationship of IL-32 and TNFalpha, we prepared an overexpression model mouse of human IL-32beta (BM-hIL-32) by bone marrow transplantation. Splenocytes of BM-hIL-32 mice showed increased expression and secretion of TNFalpha, IL-1beta, and IL-6 especially in response to lipopolysaccharide stimulation. Moreover, serum TNFalpha concentration showed a clear increase in BM-hIL-32 mice. Cell-sorting analysis of splenocytes showed that the expression of TNFalpha was increased in resting F4/80+ macrophages, and the expression of TNFalpha, IL-1beta and IL-6 was increased in lipopolysaccharide-stimulated F4/80+ macrophages and CD11c+ dendritic cells. In fact, BM-hIL-32 mice showed exacerbation of collagen-antibody-induced arthritis and trinitrobenzen sulfonic acid-induced colitis. In addition, the transfer of hIL-32beta-producing CD4+ T cells significantly exacerbated collagen-induced arthritis, and a TNFalpha blockade cancelled the exacerbating effects of hIL-32beta. We therefore conclude that IL-32 is closely associated with TNFalpha, and contributes to the exacerbation of TNFalpha-related inflammatory arthritis and colitis.
Figures


References
-
- Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, Smolen JS, Weisman M, Emery P, Feldmann M, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594–1602. doi: 10.1056/NEJM200011303432202. - DOI - PubMed
-
- Hata H, Sakaguchi N, Yoshitomi H, Iwakura Y, Sekikawa K, Azuma Y, Kanai C, Moriizumi E, Nomura T, Nakamura T, et al. Distinct contribution of IL-6, TNF-alpha, IL-1, and IL-10 to T cell-mediated spontaneous autoimmune arthritis in mice. J Clin Invest. 2004;114:582–588. doi: 10.1172/JCI200421795. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

