Interactions between drugs and occupied receptors
- PMID: 17079019
- PMCID: PMC2045509
- DOI: 10.1016/j.pharmthera.2006.08.002
Interactions between drugs and occupied receptors
Abstract
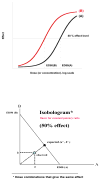
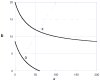
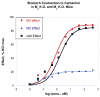
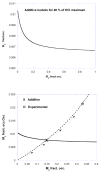
This review has 2 parts. Part I deals with isobolographic procedures that are traditionally applied to the joint action of agonists that individually produce overtly similar effects. Special attention is directed to newer computational procedures that apply to agonists with dissimilar concentration-effect curves. These newer procedures are consistent with the isobolographic methods introduced and used by Loewe, however, the present communications provides the needed graphical and mathematical detail. A major aim is distinguishing super and sub-addictive interactions from those that are simply additive. The detection and measurement of an interaction is an important step in exploring drug mechanism and is also important clinically. Part II discusses a new use of isoboles that is applicable to a single drug or chemical whose effect is mediated by 2 or more receptor subtypes. This application produces a metric that characterizes the interaction between the receptor subtypes. The expansion of traditional isobolographic theory to this multi-receptor situation follows from the newer approaches for 2-drug combination analysis in Part I. This topic leads naturally to a re-examination of competitive antagonism and the classic Schild plot. In particular, it is shown here that the Schild plot in the multi-receptor case is not necessarily linear with unit slope. Both parts of this review emphasize the quantitative aspects rather than the many drugs that have been analyzed with isobolographic methods. The mathematical exposition is rather elementary and is further aided by several graphs. An appendix is included for the reader interested in the mathematical details.
Figures







References
-
- Berenbaum MC. What is synergy? Pharmacol Rev. 1989;41:93–141. - PubMed
-
- Bolan EA, Tallarida RJ, Pasternak GW. Synergy between mu receptor subtypes: Evidence for functional interaction among mu receptor subtypes. J Pharmacol Exp Ther. 2002;303:557–562. - PubMed
-
- DiFazio CA, Brown RE, Ball CG, Heckel CG, Kennedy SS. Additive effects of anesthetics and theories of anesthesia. Anesthesiology. 1972;36:57–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

