Replicase genes of murine coronavirus strains A59 and JHM are interchangeable: differences in pathogenesis map to the 3' one-third of the genome
- PMID: 17079303
- PMCID: PMC1797483
- DOI: 10.1128/JVI.01944-06
Replicase genes of murine coronavirus strains A59 and JHM are interchangeable: differences in pathogenesis map to the 3' one-third of the genome
Abstract
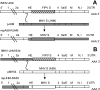
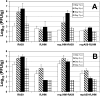
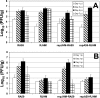
The important roles of the spike protein and other structural proteins in murine coronavirus (MHV) pathogenesis have been demonstrated; however, the role of the replicase gene remains unexplored. We assessed the influence of the replicase genes of the highly neurovirulent MHV-JHM strain and the hepatotropic and mildly neurovirulent A59 strain in acute infection of the mouse. Analysis of chimeric A59/JHM recombinant viruses indicates that the replicase genes are interchangeable and that it is the 3' end of the genome, encoding the structural proteins, rather than the replicase gene, that determines the pathogenic properties of these chimeras.
Figures

Similar articles
-
The murine coronavirus nucleocapsid gene is a determinant of virulence.J Virol. 2010 Feb;84(4):1752-63. doi: 10.1128/JVI.01758-09. Epub 2009 Dec 9. J Virol. 2010. PMID: 20007284 Free PMC article.
-
Targeted recombination within the spike gene of murine coronavirus mouse hepatitis virus-A59: Q159 is a determinant of hepatotropism.J Virol. 1998 Dec;72(12):9628-36. doi: 10.1128/JVI.72.12.9628-9636.1998. J Virol. 1998. PMID: 9811696 Free PMC article.
-
Both spike and background genes contribute to murine coronavirus neurovirulence.J Virol. 2006 Jul;80(14):6834-43. doi: 10.1128/JVI.00432-06. J Virol. 2006. PMID: 16809289 Free PMC article.
-
Murine coronavirus neuropathogenesis: determinants of virulence.J Neurovirol. 2010 Nov;16(6):427-34. doi: 10.3109/13550284.2010.529238. Epub 2010 Nov 12. J Neurovirol. 2010. PMID: 21073281 Free PMC article. Review.
-
Pathogenesis of murine coronavirus in the central nervous system.J Neuroimmune Pharmacol. 2010 Sep;5(3):336-54. doi: 10.1007/s11481-010-9202-2. Epub 2010 Apr 6. J Neuroimmune Pharmacol. 2010. PMID: 20369302 Free PMC article. Review.
Cited by
-
Genetic determinants of mouse hepatitis virus strain 1 pneumovirulence.J Virol. 2010 Sep;84(18):9278-91. doi: 10.1128/JVI.00330-10. Epub 2010 Jul 14. J Virol. 2010. PMID: 20631137 Free PMC article.
-
Protective role of Toll-like Receptor 3-induced type I interferon in murine coronavirus infection of macrophages.Viruses. 2012 May;4(5):901-23. doi: 10.3390/v4050901. Epub 2012 May 24. Viruses. 2012. PMID: 22754655 Free PMC article.
-
Cell-type-specific type I interferon antagonism influences organ tropism of murine coronavirus.J Virol. 2011 Oct;85(19):10058-68. doi: 10.1128/JVI.05075-11. Epub 2011 Jul 13. J Virol. 2011. PMID: 21752905 Free PMC article.
-
The murine coronavirus nucleocapsid gene is a determinant of virulence.J Virol. 2010 Feb;84(4):1752-63. doi: 10.1128/JVI.01758-09. Epub 2009 Dec 9. J Virol. 2010. PMID: 20007284 Free PMC article.
-
The replicase gene of avian coronavirus infectious bronchitis virus is a determinant of pathogenicity.PLoS One. 2009 Oct 9;4(10):e7384. doi: 10.1371/journal.pone.0007384. PLoS One. 2009. PMID: 19816578 Free PMC article.
References
-
- de Haan, C. A., M. de Wit, L. Kuo, C. Montalto-Morrison, B. L. Haagmans, S. R. Weiss, P. S. Masters, and P. J. Rottier. 2003. The glycosylation status of the murine hepatitis coronavirus M protein affects the interferogenic capacity of the virus in vitro and its ability to replicate in the liver but not the brain. Virology 312:395-406. - PMC - PubMed
-
- Ding, J. W., Q. Ning, M. F. Liu, A. Lai, J. Leibowitz, K. M. Peltekian, E. H. Cole, L. S. Fung, C. Holloway, P. A. Marsden, H. Yeger, M. J. Phillips, and G. A. Levy. 1997. Fulminant hepatic failure in murine hepatitis virus strain 3 infection: tissue-specific expression of a novel fgl2 prothrombinase. J. Virol. 71:9223-9230. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

