Hijacking components of the cellular secretory pathway for replication of poliovirus RNA
- PMID: 17079330
- PMCID: PMC1797456
- DOI: 10.1128/JVI.01820-06
Hijacking components of the cellular secretory pathway for replication of poliovirus RNA
Abstract
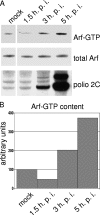
Infection of cells with poliovirus induces a massive intracellular membrane reorganization to form vesicle-like structures where viral RNA replication occurs. The mechanism of membrane remodeling remains unknown, although some observations have implicated components of the cellular secretory and/or autophagy pathways. Recently, we showed that some members of the Arf family of small GTPases, which control secretory trafficking, became membrane-bound after the synthesis of poliovirus proteins in vitro and associated with newly formed membranous RNA replication complexes in infected cells. The recruitment of Arfs to specific target membranes is mediated by a group of guanine nucleotide exchange factors (GEFs) that recycle Arf from its inactive, GDP-bound state to an active GTP-bound form. Here we show that two different viral proteins independently recruit different Arf GEFs (GBF1 and BIG1/2) to the new structures that support virus replication. Intracellular Arf-GTP levels increase approximately 4-fold during poliovirus infection. The requirement for these GEFs explains the sensitivity of virus growth to brefeldin A, which can be rescued by the overexpression of GBF1. The recruitment of Arf to membranes via specific GEFs by poliovirus proteins provides an important clue toward identifying cellular pathways utilized by the virus to form its membranous replication complex.
Figures






References
-
- Agol, V. I., G. A. Belov, K. Bienz, D. Egger, M. S. Kolesnikova, N. T. Raikhlin, L. I. Romanova, E. A. Smirnova, and E. A. Tolskaya. 1998. Two types of death of poliovirus-infected cells: caspase involvement in the apoptosis but not cytopathic effect. Virology 252:343-353. - PubMed
-
- Aldabe, R., and L. Carrasco. 1995. Induction of membrane proliferation by poliovirus proteins 2C and 2BC. Biochem. Biophys. Res. Commun. 206:64-76. - PubMed
-
- Behnia, R., and S. Munro. 2005. Organelle identity and the signposts for membrane traffic. Nature 438:597-604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

