Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels
- PMID: 17079490
- PMCID: PMC1859892
- DOI: 10.1073/pnas.0607465103
Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels
Abstract
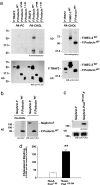
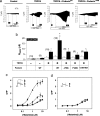
The prohibitin (PHB)-domain proteins are membrane proteins that regulate a variety of biological activities, including mechanosensation, osmotic homeostasis, and cell signaling, although the mechanism of this regulation is unknown. We have studied two members of this large protein family, MEC-2, which is needed for touch sensitivity in Caenorhabditis elegans, and Podocin, a protein involved in the function of the filtration barrier in the mammalian kidney, and find that both proteins bind cholesterol. This binding requires the PHB domain (including palmitoylation sites within it) and part of the N-terminally adjacent hydrophobic domain that attaches the proteins to the inner leaflet of the plasma membrane. By binding to MEC-2 and Podocin, cholesterol associates with ion-channel complexes to which these proteins bind: DEG/ENaC channels for MEC-2 and TRPC channels for Podocin. Both the MEC-2-dependent activation of mechanosensation and the Podocin-dependent activation of TRPC channels require cholesterol. Thus, MEC-2, Podocin, and probably many other PHB-domain proteins by binding to themselves, cholesterol, and target proteins regulate the formation and function of large protein-cholesterol supercomplexes in the plasma membrane.
Conflict of interest statement
Conflict of interest statement: Columbia University has filed a provisional patent application based on this research.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

