Activity-dependent plasticity in the olfactory intrabulbar map
- PMID: 17079653
- PMCID: PMC6674536
- DOI: 10.1523/JNEUROSCI.2805-06.2006
Activity-dependent plasticity in the olfactory intrabulbar map
Abstract
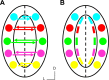
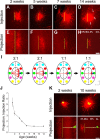
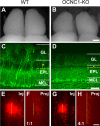
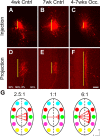
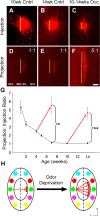
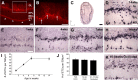
In mammals, each olfactory bulb contains two mirror-symmetric glomerular maps. Isofunctional glomeruli within each bulb are specifically linked through a set of reciprocal intrabulbar projections (IBPs) to form an intrabulbar map. We injected neural tracers into the glomerular layer on one side of the bulb and examined the resulting projection on the opposite side. In adult mice, the size of the projection tuft is directly proportional to the size of the injected region. Using this ratio as a measure of IBP maturity, we find an immature 5:1 projection to injection ratio at 1 week of age that gradually refines to a mature 1:1 by 7 weeks. Moreover, whereas the glomerular map is able to form despite the elimination of odorant-induced activity, the intrabulbar map shows clear activity dependence for its precise formation. Here we show through experiments with both naris-occluded and anosmic mice that odorant-induced activity is not required to establish IBPs but is crucial for projection refinement. In contrast, increased glomerular activation through exposure to distinct odorants during map development can accelerate the refinement of projections associated with the activated glomeruli. These findings illustrate a clear role for odorant-induced activity in shaping the internal circuitry of the bulb. Interestingly, activity deprivation can alter the organization of both the developing and the mature map to the same degree, demonstrating that intrabulbar map plasticity is maintained into adulthood with no discernible critical period.
Figures


References
-
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. - PubMed
-
- Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in G(olf) are anosmic. Neuron. 1998;20:69–81. - PubMed
-
- Belluscio L, Lodovichi C, Feinstein P, Mombaerts P, Katz LC. Odorant receptors instruct functional circuitry in the mouse olfactory bulb. Nature. 2002;419:296–300. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
