Increased citrullination of histone H3 in multiple sclerosis brain and animal models of demyelination: a role for tumor necrosis factor-induced peptidylarginine deiminase 4 translocation
- PMID: 17079667
- PMCID: PMC6674531
- DOI: 10.1523/JNEUROSCI.3349-06.2006
Increased citrullination of histone H3 in multiple sclerosis brain and animal models of demyelination: a role for tumor necrosis factor-induced peptidylarginine deiminase 4 translocation
Abstract
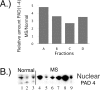
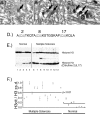
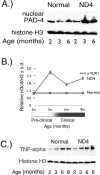
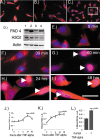
Modification of arginine residues by citrullination is catalyzed by peptidylarginine deiminases (PADs), of which five are known, generating irreversible protein structural modifications. We have shown previously that enhanced citrullination of myelin basic protein contributed to destabilization of the myelin membrane in the CNS of multiple sclerosis (MS) patients. We now report increased citrullination of nucleosomal histones by PAD4 in normal-appearing white matter (NAWM) of MS patients and in animal models of demyelination. Histone citrullination was attributable to increased levels and activity of nuclear PAD4. PAD4 translocation into the nucleus was attributable to elevated tumor necrosis factor-alpha (TNF-alpha) protein. The elevated TNF-alpha in MS NAWM was not associated with CD3+ or CD8+ lymphocytes, nor was it associated with CD68+ microglia/macrophages. GFAP, a measure of astrocytosis, was the only cytological marker that was consistently elevated in the MS NAWM, suggesting that TNF-alpha may have been derived from astrocytes. In cell cultures of mouse and human oligodendroglial cell lines, PAD4 was predominantly cytosolic but TNF-alpha treatment induced its nuclear translocation. To address the involvement of TNF-alpha in targeting PAD4 to the nucleus, we found that transgenic mice overexpressing TNF-alpha also had increased levels of citrullinated histones and elevated nuclear PAD4 before demyelination. In conclusion, high citrullination of histones consequent to PAD4 nuclear translocation is part of the process that leads to irreversible changes in oligodendrocytes and may contribute to apoptosis of oligodendrocytes in MS.
Figures

References
-
- Akassoglou K, Probert L, Kontogeorgos G, Kollias G. Astrocyte-specific but not neuron-specific transmembrane TNF triggers inflammation and degeneration in the central nervous system of transgenic mice. J Immunol. 1997;158:438–445. - PubMed
-
- Akassoglou K, Bauer J, Kassiotis G, Pasparakis M, Lassmann H, Kollias G, Probert L. Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice: models for multiple sclerosis with primary oligodendrogliopathy. Am J Pathol. 1998;153:801–813. - PMC - PubMed
-
- Akassoglou K, Bauer J, Kassiotis G, Lassmann H, Kollias G, Probert L. Transgenic models of TNF induced demyelination. Adv Exp Med Biol. 1999;468:245–259. - PubMed
-
- Akiyama K, Sakurai Y, Asou H, Senshu T. Localization of peptidylarginine deiminase type II in a stage-specific immature oligodendrocyte from rat cerebral hemisphere. Neurosci Lett. 1999;274:53–55. - PubMed
-
- Brooks WH. Autoimmune disorders result from loss of epigenetic control following chromosome damage. Med Hypotheses. 2005;64:590–598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
