The vacuolar transporter chaperone (VTC) complex is required for microautophagy
- PMID: 17079729
- PMCID: PMC1751332
- DOI: 10.1091/mbc.e06-08-0664
The vacuolar transporter chaperone (VTC) complex is required for microautophagy
Abstract
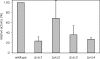
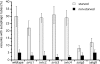
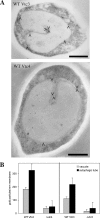
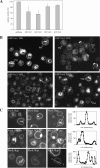
Microautophagy involves direct invagination and fission of the vacuolar/lysosomal membrane under nutrient limitation. This occurs by an autophagic tube, a specialized vacuolar membrane invagination that pinches off vesicles into the vacuolar lumen. In this study we have identified the VTC (vacuolar transporter chaperone) complex as required for microautophagy. The VTC complex is present on the ER and vacuoles and at the cell periphery. On induction of autophagy by nutrient limitation the VTC complex is recruited to and concentrated on vacuoles. The VTC complex is inhomogeneously distributed within the vacuolar membranes, showing an enrichment on autophagic tubes. Deletion of the VTC complex blocks microautophagic uptake into vacuoles. The mutants still form autophagic tubes but the production of microautophagic vesicles from their tips is impaired. In line with this, affinity-purified antibodies to the Vtc proteins inhibit microautophagic uptake in a reconstituted system in vitro. Our data suggest that the VTC complex is an important constituent of autophagic tubes and that it is required for scission of microautophagic vesicles from these tubes.
Figures



References
-
- Babu Y. S., Sack J. S., Greenhough T. J., Bugg C. E., Means A. R., Cook W. J. Three-dimensional structure of calmodulin. Nature. 1985;315:37–40. - PubMed
-
- Bachs O., Lanini L., Serratosa J., Coll M. J., Bastos R., Aligue R., Rius E., Carafoli E. Calmodulin-binding proteins in the nuclei of quiescent and proliferatively activated rat liver cells. J. Biol. Chem. 1990;265:18595–18600. - PubMed
-
- Bergamini E., Cavallini G., Donati A., Gori Z. The anti-ageing effects of caloric restriction may involve stimulation of macroautophagy and lysosomal degradation, and can be intensified pharmacologically. Biomed. Pharmacother. 2003;57:203–208. - PubMed
-
- Burgoyne R. D., Clague M. J. Calcium and calmodulin in membrane fusion. Biochim. Biophys. Acta. 2003;1641:137–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

