Mitochondrial localization and function of heme oxygenase-1 in cigarette smoke-induced cell death
- PMID: 17079780
- PMCID: PMC1899328
- DOI: 10.1165/rcmb.2006-0214OC
Mitochondrial localization and function of heme oxygenase-1 in cigarette smoke-induced cell death
Retraction in
-
Retraction: Mitochondrial Localization and Function of Heme Oxygenase-1 in Cigarette Smoke-induced Cell Death.Am J Respir Cell Mol Biol. 2023 Apr;68(4):463. doi: 10.1165/rcmb.6804Retraction. Am J Respir Cell Mol Biol. 2023. PMID: 37000443 Free PMC article. No abstract available.
Abstract
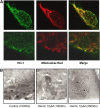
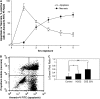
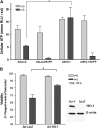
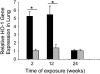
Cigarette smoke-induced apoptosis and necrosis contribute to the pathogenesis of chronic obstructive pulmonary disease. The induction of heme oxygenase-1 provides cytoprotection against oxidative stress, and may protect in smoking-related disease. Since mitochondria regulate cellular death, we examined the functional expression and mitochondrial localization of heme oxygenase-1 in pulmonary epithelial cells exposed to cigarette smoke extract (CSE), and its role in modulating cell death. Heme oxygenase-1 expression increased dramatically in cytosolic and mitochondrial fractions of human alveolar (A549), or bronchial epithelial cells (Beas-2b) exposed to either hemin, lipopolysaccharide, or CSE. Mitochondrial localization of heme oxygenase-1 was also observed in a primary culture of human small airway epithelial cells. Furthermore, heme oxygenase activity increased dramatically in mitochondrial fractions, and in whole cell extracts of Beas-2b after exposure to hemin and CSE. The mitochondrial localization of heme oxygenase-1 in Beas-2b was confirmed using immunogold-electron microscopy and immunofluorescence labeling on confocal laser microscopy. CSE caused loss of cellular ATP and rapid depolarization of mitochondrial membrane potential. Apoptosis occurred in Beas-2b at low concentrations of cigarette smoke extract, whereas necrosis occurred at high concentrations. Overexpression of heme oxygenase-1 inhibited CSE-induced Beas-2b cell death and preserved cellular ATP levels. Finally, heme oxygenase-1 mRNA expression was elevated in the lungs of mice chronically exposed to cigarette smoke. We demonstrate the functional compartmentalization of heme oxygenase-1 in the mitochondria of lung epithelial cells, and its potential role in defense against mitochondria-mediated cell death during CSE exposure.
Figures
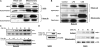
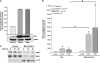
References
-
- Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004;364:613–620. - PubMed
-
- Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 2003;22:672–688. - PubMed
-
- Rahman I, MacNee W. Role of oxidants/antioxidants in smoking-induced lung diseases. Free Radic Biol Med 1996;21:669–681. - PubMed
-
- Rustemeier K, Stabbert R, Haussmann HJ, Roemer E, Carmines EL. Evaluation of the potential effects of ingredients added to cigarettes. Part 2: chemical composition of mainstream smoke. Food Chem Toxicol 2002;40:93–104. - PubMed
-
- Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001;163:1256–1276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

