Phosphorylation-dependent ubiquitination of cyclin D1 by the SCF(FBX4-alphaB crystallin) complex
- PMID: 17081987
- PMCID: PMC1702390
- DOI: 10.1016/j.molcel.2006.09.007
Phosphorylation-dependent ubiquitination of cyclin D1 by the SCF(FBX4-alphaB crystallin) complex
Abstract
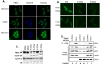
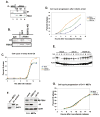
Growth factor-dependent accumulation of the cyclin D1 proto-oncogene is balanced by its rapid phosphorylation-dependent proteolysis. Degradation is triggered by threonine 286 phosphorylation, which promotes its ubiquitination by an unknown E3 ligase. We demonstrate that Thr286-phosphorylated cyclin D1 is recognized by a Skp1-Cul1-F box (SCF) ubiquitin ligase where FBX4 and alphaB crystallin govern substrate specificity. Overexpression of FBX4 and alphaB crystallin triggered cyclin D1 ubiquitination and increased cyclin D1 turnover. Impairment of SCF(FBX4-alphaB crystallin) function attenuated cyclin D1 ubiquitination, promoting cyclin D1 overexpression and accelerated cell-cycle progression. Purified SCF(FBX4-alphaB crystallin) catalyzed polyubiquitination of cyclin D1 in vitro. Consistent with a putative role for a cyclin D1 E3 ligase in tumorigenesis, FBX4 and alphaB crystallin expression was reduced in tumor-derived cell lines and a subset of primary human cancers that overexpress cyclin D1. We conclude that SCF(FBX4-alphaB crystallin) is an E3 ubiquitin ligase that promotes ubiquitin-dependent degradation of Thr286-phosphorylated cyclin D1.
Figures
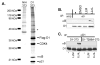




Similar articles
-
SCF(Fbx4/alphaB-crystallin) E3 ligase: when one is not enough.Cell Cycle. 2008 Oct;7(19):2983-6. doi: 10.4161/cc.7.19.6775. Epub 2008 Oct 12. Cell Cycle. 2008. PMID: 18818515 Review.
-
Lysine 269 is essential for cyclin D1 ubiquitylation by the SCF(Fbx4/alphaB-crystallin) ligase and subsequent proteasome-dependent degradation.Oncogene. 2009 Dec 10;28(49):4317-25. doi: 10.1038/onc.2009.287. Oncogene. 2009. PMID: 19767775 Free PMC article.
-
Phosphorylation-dependent regulation of SCF(Fbx4) dimerization and activity involves a novel component, 14-3-3ɛ.Oncogene. 2011 Apr 28;30(17):1995-2002. doi: 10.1038/onc.2010.584. Epub 2011 Jan 17. Oncogene. 2011. PMID: 21242966 Free PMC article.
-
The small heat-shock protein alpha B-crystallin promotes FBX4-dependent ubiquitination.J Biol Chem. 2003 Feb 14;278(7):4699-704. doi: 10.1074/jbc.M211403200. Epub 2002 Dec 4. J Biol Chem. 2003. PMID: 12468532
-
The Biology of F-box Proteins: The SCF Family of E3 Ubiquitin Ligases.Adv Exp Med Biol. 2020;1217:111-122. doi: 10.1007/978-981-15-1025-0_8. Adv Exp Med Biol. 2020. PMID: 31898225 Review.
Cited by
-
Cyclin D1, cancer progression, and opportunities in cancer treatment.J Mol Med (Berl). 2016 Dec;94(12):1313-1326. doi: 10.1007/s00109-016-1475-3. Epub 2016 Oct 2. J Mol Med (Berl). 2016. PMID: 27695879 Free PMC article. Review.
-
The role of small heat-shock protein αB-crystalline (HspB5) in COPD pathogenesis.Int J Chron Obstruct Pulmon Dis. 2012;7:633-40. doi: 10.2147/COPD.S34929. Epub 2012 Oct 4. Int J Chron Obstruct Pulmon Dis. 2012. PMID: 23055712 Free PMC article.
-
Overexpression of Fbxo6 inactivates spindle checkpoint by interacting with Mad2 and BubR1.Cell Cycle. 2018;17(24):2779-2789. doi: 10.1080/15384101.2018.1557488. Epub 2018 Dec 18. Cell Cycle. 2018. PMID: 30526252 Free PMC article.
-
The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention.Mol Cancer. 2007 Apr 2;6:24. doi: 10.1186/1476-4598-6-24. Mol Cancer. 2007. PMID: 17407548 Free PMC article. Review.
-
Nuclear accumulation of cyclin D1 during S phase inhibits Cul4-dependent Cdt1 proteolysis and triggers p53-dependent DNA rereplication.Genes Dev. 2007 Nov 15;21(22):2908-22. doi: 10.1101/gad.1586007. Genes Dev. 2007. PMID: 18006686 Free PMC article.
References
-
- Alt JR, Gladden AB, Diehl JA. p21(Cip1) Promotes cyclin D1 nuclear accumulation via direct inhibition of nuclear export. J Biol Chem. 2002;277:8517–8523. - PubMed
-
- Bai F, Xi JH, Wawrousek EF, Fleming TP, Andley UP. Hyperproliferation and p53 status of lens epithelial cells derived from alphaB-crystallin knockout mice. J Biol Chem. 2003;278:36876–36886. - PubMed
-
- Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature. 2004;428:190–193. - PubMed
-
- Bax B, Carter PS, Lewis C, Guy AR, Bridges A, Tanner R, Pettman G, Mannix C, Culbert AA, Brown MJ, et al. The structure of phosphorylated GSK-3beta complexed with a peptide, FRATtide, that inhibits beta-catenin phosphorylation. Structure (Camb) 2001;9:1143–1152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

