Cellular mechanisms of burst firing-mediated long-term depression in rat neocortical pyramidal cells
- PMID: 17082228
- PMCID: PMC2075152
- DOI: 10.1113/jphysiol.2006.123588
Cellular mechanisms of burst firing-mediated long-term depression in rat neocortical pyramidal cells
Abstract
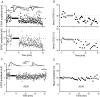
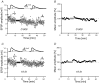
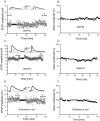
During wakefulness and sleep, neurons in the neocortex emit action potentials tonically or in rhythmic bursts, respectively. However, the role of synchronized discharge patterns is largely unknown. We have recently shown that pairings of excitatory postsynaptic potentials (EPSPs) and action potential bursts or single spikes lead to long-term depression (burst-LTD) or long-term potentiation, respectively. In this study, we elucidate the cellular mechanisms of burst-LTD and characterize its functional properties. Whole-cell patch-clamp recordings were obtained from layer V pyramidal cells in somatosensory cortex of juvenile rats in vitro and composite EPSPs and EPSCs were evoked extracellularly in layers II/III. Repetitive burst-pairings led to a long-lasting depression of EPSPs and EPSCs that was blocked by inhibitors of metabotropic glutamate group 1 receptors, phospholipase C, protein kinase C (PKC) and calcium release from the endoplasmic reticulum, and that required an intact machinery for endocytosis. Thus, burst-LTD is induced via a Ca2+- and phosphatidylinositol-dependent activation of PKC and expressed through phosphorylation-triggered endocytosis of AMPA receptors. Functionally, burst-LTD is inversely related to EPSP size and bursts dominate single spikes in determining the sign of synaptic plasticity. Thus burst-firing constitutes a signal by which coincident synaptic inputs are proportionally downsized. Overall, our data thus suggest a mechanism by which synaptic weights can be reconfigured during non-rapid eye movement sleep.
Figures
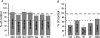


References
-
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Rev. 1999;366:151–158. - PubMed
-
- Artola A, Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 1993;16:480–487. - PubMed
-
- Batchelor AM, Garthwaite J. Frequency detection and temporally dispersed synaptic signal association through a metabotropic glutamate receptor pathway. Nature. 1997;385:74–77. - PubMed
-
- Charpak S, Gähwiler B. Glutamate mediates a slow synaptic repsonse in hippocampal slices cultures. Proc R Soc Lond B Biol Sci. 1991;243:221–226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

