Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation
- PMID: 17082319
- PMCID: PMC1801044
- DOI: 10.1182/blood-2006-08-041368
Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation
Abstract
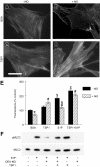
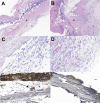
The nitric oxide (NO)/cGMP pathway, by relaxing vascular smooth muscle cells, is a major physiologic regulator of tissue perfusion. We now identify thrombospondin-1 as a potent antagonist of NO for regulating F-actin assembly and myosin light chain phosphorylation in vascular smooth muscle cells. Thrombospondin-1 prevents NO-mediated relaxation of precontracted vascular smooth muscle cells in a collagen matrix. Functional magnetic resonance imaging demonstrated that an NO-mediated increase in skeletal muscle perfusion was enhanced in thrombospondin-1-null relative to wild-type mice, implicating endogenous thrombospondin-1 as a physiologic antagonist of NO-mediated vasodilation. Using a random myocutaneous flap model for ischemic injury, tissue survival was significantly enhanced in thrombospondin-1-null mice. Improved flap survival correlated with increased recovery of oxygen levels in the ischemic tissue of thrombospondin-1-null mice as measured by electron paramagnetic resonance oximetry. These findings demonstrate an important antagonistic relation between NO/cGMP signaling and thrombospondin-1 in vascular smooth muscle cells to regulate vascular tone and tissue perfusion.
Figures




References
-
- Brevetti G, Chiariello M. Peripheral arterial disease: the magnitude of the problem and its socioeconomic impact. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:199–208. - PubMed
-
- Strandness DE, Jr, Eidt JF. Peripheral vascular disease. Circulation. 2000;102:IV46–IV51. - PubMed
-
- Heller L, Levin LS. Lower extremity microsurgical reconstruction. Plast Reconstr Surg. 2001;108:1029–1041. quiz 1042. - PubMed
-
- Kuwahara M, Tada H, Mashiba K, et al. Mortality and recurrence rate after pressure ulcer operation for elderly long-term bedridden patients. Ann Plast Surg. 2005;54:629–632. - PubMed
-
- Castelli ML, Pecorari G, Succo G, Bena A, Andreis M, Sartoris A. Pectoralis major myocutaneous flap: analysis of complications in difficult patients. Eur Arch Otorhinolaryngol. 2001;258:542–545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

