Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype
- PMID: 17082491
- PMCID: PMC1868577
- DOI: 10.1161/01.ATV.0000251500.94478.18
Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype
Erratum in
- Arterioscler Thromb Vasc Biol. 2007 Feb;27(2):e8. Schmidt, Harald H H [added]
Abstract
Objective: The mechanisms responsible for maintaining the differentiated phenotype of adult vascular smooth muscle cells (VSMCs) are incompletely understood. Reactive oxygen species (ROS) have been implicated in VSMC differentiation, but the responsible sources are unknown. In this study, we investigated the role of Nox1 and Nox4-derived ROS in this process.
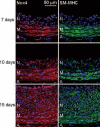
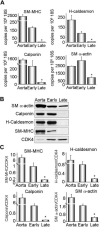
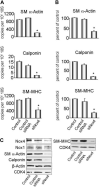
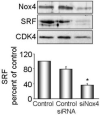
Methods and results: Primary VSMCs were used to study the relationship between Nox homologues and differentiation markers such as smooth muscle alpha-actin (SM alpha-actin), smooth muscle myosin heavy chain (SM-MHC), heavy caldesmon, and calponin. We found that Nox4 and differentiation marker genes were downregulated from passage 1 to passage 6 to 12, whereas Nox1 was gradually upregulated. Nox4 co-localized with SM alpha-actin-based stress fibers in differentiated VSMC, and moved into focal adhesions in de-differentiated cells. siRNA against nox4 reduced NADPH-driven superoxide production in serum-deprived VSMCs and downregulated SM-alpha actin, SM-MHC, and calponin, as well as SM-alpha actin stress fibers. Nox1 depletion did not decrease these parameters.
Conclusions: Nox4-derived ROS are critical to the maintenance of the differentiated phenotype of VSMCs. These findings highlight the importance of identifying the specific source of ROS involved in particular cellular functions when designing therapeutic interventions.
Figures


Comment in
-
Nox 4 regulation of vascular smooth muscle cell differentiation marker gene expression.Arterioscler Thromb Vasc Biol. 2007 Jan;27(1):12-4. doi: 10.1161/01.ATV.0000254154.43871.50. Arterioscler Thromb Vasc Biol. 2007. PMID: 17185622 No abstract available.
References
-
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. - PubMed
-
- Rovner AS, Murphy RA, Owens GK. Expression of smooth muscle and nonmuscle myosin heavy chains in cultured vascular smooth muscle cells. J Biol Chem. 1986;261:14740–14745. - PubMed
-
- Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42:1075–1081. - PubMed
-
- Su B, Mitra S, Gregg H, Flavahan S, Chotani MA, Clark KR, Goldschmidt-Clermont PJ, Flavahan NA. Redox regulation of vascular smooth muscle cell differentiation. Circ Res. 2001;89:39–46. - PubMed
-
- Jin L, Ying Z, Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol. 2004;287:H1495–H1500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

